Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research - (2019)Volume 9, Issue 7
Objective: In women hypersensitive to aggressive detergents or easily developing genital dryness, the daily feminine hygiene should require caution in adopting an adequate product to avoid the onset or the impairment of an inflammatory condition, still maintaining high protection against infection. A new intimate cleanser, called Saugella Acti3 (SA3), containing antimicrobial ingredients (thymol and Zinc) with mild non-aggressive natural surfactants (derived from coconut and the amino acids of wheat) and mucoadhesive agents (xanthan gum and carrageenan) was formulated. Zinc potentiates antimicrobial, anti-inflammatory and antioxidant action of thymol, and the mucoadhesive system increases the permanence in situ of the active ingredients, so prolonging their effect. Natural surfactants stimulate an epidermal renewal of keratinocytes, restructuring the damaged skin and restoring the cutaneous lipid layer removed during washing. The study evaluated the clinical effect of SA3 in a controlled randomized study versus a standard cleanser in fertile age women more exposed to infection risk condition and hypersensitive to aggressive detergents.
Material and Methods: Women of childbearing potential needing effective intimate hygiene in specific life periods (menstrual cycle, pregnancy or post-partum and infection risk conditions) were enrolled in a double-blind, controlled, parallel-group, randomized study. The women were assigned to the treatment with SA3 (Saugella Acti3, Mylan) a standard detergent for feminine care, twice daily for 4 weeks. Clinical evaluations were performed at baseline, 2 and 4 weeks.
Results: Forty-eight women, 24 per treatment, mean age 31.9 years, were treated. Symptoms severity was significantly reduced versus baseline with SA3, compared to what observed with I: for itching 42.0% on SA3 vs +20.0% on I), burning (-35.5% vs +85.2%), dyspareunia (-63.6% vs +42.9%).
At the end of the treatment, SA3 was superior to I: women felt significantly better vs baseline 70.8% vs 8.3%, the overall condition improved in 66.7% vs 12.5%, and the availability to continue the treatment was confirmed by 83.3% vs 41.7%.
Conclusion: The combination of microbiologically active ingredients, whose action is prolonged by mucoadhesive components even after cleansing, supported by natural non-aggressive surfactants, allowed a better clinical result than a standard detergent in intimate hygiene in infection risk conditions.
Feminine hygiene; Vulvo-vaginal protection; Vaginal dryness; Plant extracts; Zinc
The vulvovaginal microbiome needs to be protected from inflammatory and infective conditions by potentiating the physiological barriers. A correct daily lifestyle should be implemented by cleansers for intimate hygiene containing microbiologically active components, chemically non-aggressive surfactants and ingredients able to retain the product by the application site, prolonging the persistence of its action in the genital area. Moreover, the current legislate requires scientific evidence supporting the activity claims for cosmetic products [1].
The cosmetic research and development fine-tuned a specific intimate cleanser of the last generation characterized by natural antibacterial, antimycotic, antioxidant and anti-inflammatory action, with mucoadhesive properties and soft surfactants, indicated for women in post-partum, pregnancy, menstrual cycle or in risk condition for genital area infections, and prone to genital non-infective irritations or dryness, consequent to normal aggressive cleansers or oral contraceptives use, in which genital mucosae can develop dryness linked to the low estrogen levels, with consequent difficulty at intercourses and more local infections.
The rationale applied to develop this new and innovative cleanser (SA3) was to increase antimicrobial and antiinflammatory activity and prolong the bioadhesive of an existing active product (SA) based on Thymus vulgaris extract at acid Ph [1-4].
Zinc Coceth Sulfate was added to SA for potentiating the antimicrobial action of thymol, and an in-vitro study confirmed the increased potency on candida spp, especially against Candida glabrata, of SA3 compared with S [1]. Moreover, the persistence on vulvo-vaginal mucosa of the active ingredients of SA3 after cleansing was extended by a mucoadhesive system combining xanthan gum with lambda carrageenan from Chondrus crispus, allowing the vulvo-vaginal high mucoadhesion of the active ingredients, remaining highly concentrated even when the formulation is progressively diluted [1].
The test formulation was completed by mild non-aggressive natural surfactants (derived from coconut and from the amino acids of wheat) stimulating an epidermal renewal of keratinocytes and, therefore, capable to restructure the damaged skin and restore the cutaneous lipid layer removed during washing. Moreover, SA3 contains hydrating and emollient substances as propylene glycol, glycerine, caprylyl glycol, and glyceryl oleate, that in synergy with the natural surfactants, can assure a particularly soft cleansing and prolonged hydration.
The objective of the study was to evaluate the clinical effect and safety of SA3 in a controlled randomized study versus a standard cleanser in women of childbearing potential more exposed to vulvo-vaginal microbial infections and hypersensitive to aggressive detergents or easily developing genital dryness.
Women of childbearing potential needing to maintain the vulvovaginal balance through effective intimate hygiene in specific reproductive life periods: menstrual cycle, pregnancy or post-partum and microbial infection risk conditions (surgical interventions, or attendance of hygienically not adequate environments) were enrolled in a double-blind, controlled, parallel-group, randomized study. Women needing concomitant treatments that could interfere with the vulvo-vaginal condition were excluded. A random number generator system was used to generate the random allocation sequence. The women consecutively referring to the gynecologist’s observation for a routine check of good health, and responding to the entry criteria, were randomly assigned to the treatment with SA3 (Saugella Acti3, Mylan) or with I (a commercially available standard detergent for feminine care, (Infasil Intimo Neutro Protezione Quotidiana). Neither the investigators nor the women were aware of the assigned intervention, and the gynecologist was provided a sealed code, to be opened only at the end of last subject’s treatment. The products were supplied in the undistinguishable package and used twice daily (morning and evening) for 4 weeks. The study was carried out in accordance with the Code of Ethics of the Declaration of Helsinki. Clinical evaluations were performed at baseline, 2 and 4 weeks.
Each subject received the product specified by the randomization list and a diary for the daily recording of signs and symptoms that was returned 4 weeks later.
The presence and severity of itching, burning, vulvovaginal erythema and edema, vaginal dryness and dyspareunia were evaluated using a semi-quantitative score between 0 and 4, where 0=absent, 1=mild, 2=moderate, 3=intense, 4=very intense. The vaginal discharge was scored as 0=absence; 1=low amount, liquid consistency, clear color; 2=visible amount, semi-dense consistency, white color; 3=bulky, dense consistency, white color.
At the end of treatment, the doctor and each woman gave an overall rating, respectively on the treatment effect (0=no, 1=poor, 2=reasonable, 3=good, 4=excellent), and on the condition compared with pre-treatment (improved, unchanged and deteriorated).
The sample size was calculated using the Simple Interactive Statistical Analysis [1]. Reduction in the overall vulvovaginal symptoms was the primary endpoint.
In a previous clinical study on a similar formulation with a lower antimicrobial and mucoadhesive than SA3, the mean severity of the vulvovaginal symptoms decreased by 64% vs 28% for the controls [1]. For the present study, a percent reduction in symptoms severity of 75% for SA3 and 28% for the alternative treatment was hypothesized. Setting α=0.05 (double-sided) and power of 1-β=0.8, the sample size calculation resulted in 17 subjects per treatment group (approximate number of cases necessary because both percentages are significant with a 5% probability level).
Analysis of variance for parametric and non-parametric data on absolute changes, 2 test for frequency data and Fisher exact test, using Excel statistical software were performed. The significance level was taken to be α=0.05 (type 1 error) and power β=0.90 (type 2 error).
Forty-eight women, 24 per treatment, mean age (SD) 31.9 years (8.6), and more precisely 32.4 years (8.5) with SA3 and 31.4 years (8.5) with C were treated.
The sample was made up of hypersensitive to normal detergents women who were at risk conditions of vulvovaginal infections or post-partum (58.3%-41.7% for SA3 and 54.2%-45.8% for I, respectively).
The two groups were homogeneous for the initial severity of the assessment parameters (Table 1).
| SA3 | I | |
|---|---|---|
| n | 24 | 24 |
| Age (yrs ± SD) | 32.4 ± 8.9 | 31.4 ± 8.5 |
| Ethnic group | Caucasian | Caucasian |
| Risk conditions | 14 | 13 |
| Post-partum | 10 | 11 |
| Itching | 1.36 ± 0.17 | 1.00 ± 0.15 |
| Burning | 1.37 ± 0.21 | 0.88 ± 0.13 |
| Oedema | 0.13 ± 0.07 | 0.13 ± 0.07 |
| Erythema | 0.08 ± 0.05 | 0.21 ± 0.10 |
| Vulvo-vaginal dryness | 1.36 ± 0.17 | 1.00 ± 0.01 |
| Dyspareunia | 1.47 ± 0.26 | 1.17 ± 0.31 |
Table 1: Characteristics and severity of symptoms at the start of treatment in the two study groups (mean ± SE).
The severity of symptoms gradually felt during the course of the treatment with a statistically significant difference in favour of SA3 versus controls after 4 week for itching (SA3 from 1.36 ± 0.17 to 0.79 ± 0.19 vs I from 1.00 ± 0.15 to 1.20 ± 0.2, p<0.01 between treatments), burning (SA3 from 1.37 ± 0.21 to 0.95 ± 0.25 vs I from 0.88 ± 0.13 to 1.63 ± 0.29, p<0.01), dryness (SA3 from 1.36 ± 0.17 to 071 ± 0.16 vs I from 1.00 ± 0.00 to 0.71 ± 0.25) and dyspareunia (SA3 from 1.47 ± 0.26 to 0.53 ± 0.13 vs I from 1.17 ± 0.31 to 1.67 ± 0.61, p<0.01) (Figures 1 and 2).
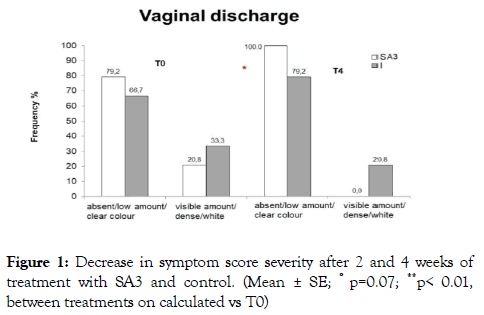
Figure 1. Decrease in symptom score severity after 2 and 4 weeks of treatment with SA3 and control. (Mean ± SE; ° p=0.07; **p< 0.01, between treatments on calculated vs T0)
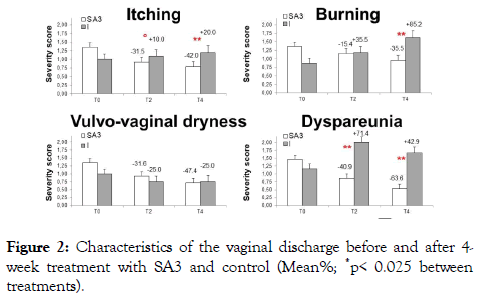
Figure 2. Characteristics of the vaginal discharge before and after 4- week treatment with SA3 and control (Mean%; *p< 0.025 between treatments).
Vulvovaginal erythema and edema, when present at baseline, disappeared in all cases in SA3 group only (3 cases) and persisted in I group (3 cases).
At the end of the treatment, physician ’ s final judgment, woman ’ s overall condition, and feeling, and availability to continue the treatment were significantly in favor of the SA3 group (p=0.01) (Figure 3).
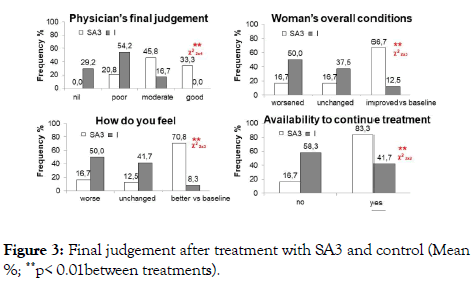
Figure 3. Final judgement after treatment with SA3 and control (Mean %; **p< 0.01between treatments).
The outcomes of the present randomized controlled trial indicate that SA3, a combination of microbiologically active ingredients, whose action is prolonged by mucoadhesive components even after cleansing, supported by natural nonaggressive surfactants, significantly improved severity of the vulvo-vaginal symptoms versus a standard cleanser in intimate hygiene of childbearing age women in infection risk conditions.
SA3 reached effective protection from microbial contamination improving in the meantime genital tissue hydration in women with vulvovaginal dryness or hypersensitive to standard cleansers used in feminine care.
Drawing an inference from these clinical data, the prevention of the vaginal irritation following the menstrual cycle, the reduction of the relapse rate in women with recurrent candidiasis, and the improvement of dyspareunia can be expected. Further study should be properly designed on these specific targets.
SA3 can be considered a medicated cosmetic, being supported by microbiological and pharmacological evidence.
This new formulation is appropriate for women looking for hydration in intimate hygiene, to avoid dryness and/or irritation of genital area usually occurring after the use of normal aggressive detergents in predisposed women.
The antimicrobial activity of SA3 is potentiated by the combination of thymol, zinc and caprylyl glycol.
The antibacterial, antimycotic, anti-inflammatory and antioxidant actions of thymol do not interfere with lactobacilli growth which contributes to a healthy environment for vaginal epithelial cells [1-5].
Thymol induces morpho-structural damages of the Candida envelope, inhibits C. albicans filamentation and the adhesion of E. coli, S. aureus, G. vaginalis and C. albicans to human vaginal cells, and antagonizes Candida and G. vaginalis biofilms [5,6].
Zinc increases antimicrobial potency of SA3, improving the minimum inhibitory concentration for Candida glabrata, that is more aggressive than C. albicans. Zinc inhibits the growth of Chlamydia trachomatis, Trichomonas vaginalis and E. coli and inactivates herpes simplex virus. Caprylyl glycol, a sebostatic and skin-purifying agent, has proven action on Gram-positive and Gram-negative micro-organisms and yeasts. At last, the acid pH of the solution completes the protection/prevention of genital infections, fungi included, favoring the lactobacilli growth and activity, as they subtract the nutritive substrates to pathogens, competing with them for the adhesion receptors and producing inhibiting factors.
The innovative factor that enriched the formulation is mucoadhesion obtained including macromolecules, as Xanthan gum and Chondrus crispus, expressing mucoadhesive interactions between the active ingredients and the tissue [4]. In this way, the permanence of the product in situ is protected by removal, significantly prolonging the antimicrobial and antiinflammatory activity of the components. The prolonged contact of thymol with the vulvo-vaginal mucosa, notwithstanding the progressive dilution of the formulation, can further guarantee reliable protection of the genital area.
The second important feature for intimate cleansers is the absence of aggressive surfactants responsible for an excessive elimination of the membrane sebum layer, whose role is to protect the structural integrity of vulvovaginal mucosa from exposing it to physical (microtraumas), chemical (soaps or other allergens) or infective attacks, so reducing vaginal dryness and dyspareunia. The innovative non-aggressive natural surfactants in SA3 permit greater hydration of the external genitals reinforcing the skin barrier. Zinc has anti-irritant action and supports collagen synthesis, fibroblast proliferation, and repair processes so favoring epidermis maturation and co-operating in keeping the skin in healthy conditions [4-30].
SA3 showed no pro-sensitizing activity in a specific safety study on the immune response [4].
In conclusion, SA3 has properties that make it suitable for effective and safe use in intimate feminine hygiene, aimed to assure the daily protection of genital wellbeing, whose compliance should minimize antibiotic resistance, and as a complementary treatment of anti-infective therapy.
The claims concerning SA3 reviewed here are consistent and reliable, and the presence of active ingredients with antibacterial, antifungal and anti-inflammatory activities can be useful in maintaining the balance of the vaginal ecosystem. Doctors should ask for scientific evidence when cosmetic are recommended for their use in daily practice.
Benvenuti C and Gasparri F are consultants and Zanardi A is employed for Mylan. No competing financial interests exist.
The supplies for the study were provided by Mylan.
Citation: Cappelli V, Benvenuti C, Gasparri F, Zanardi A, De Leo V (2019) Evidence on a New Thymol and Zinc-Based Cleanser for Feminine Hygiene in a Double-Blind Controlled Randomized Study. Gynecol Obstet (Sunnyvale) 9:508. doi: 10.24015/2161-10932.19.9.508
Received: 03-Jul-2019 Accepted: 08-Jul-2019 Published: 15-Jul-2019
Copyright: © 2019 Cappelli V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Sources of funding : The supplies for the study were provided by Mylan.