Research Article - (2023)Volume 9, Issue 2
Clinical Examples of the Side-Effects of COVID-19 Vaccines, the Good, the Bad and the Unexpected: A Hong Kong Study
John SM Leung*Abstract
This is a front-line clinical study on the side-effects of two COVID-19 vaccines encountered in a hospital-based combined outpatient and inpatient clinical practice. The subjects include 15 patients almost evenly distributed between the sexes, and incurring 24 incidents of side effects, of which 15 were considered deleterious, 4 were (surprisingly) beneficial and five were considered having some possible beneficial effects. The deleterious side effects of COVID-19 vaccines include pain (ranging from pain in injection site to myalgia, arthralgia and neuralgia), fever, diarrhoea (including flare up of occult chronic intestinal infection), fatigue, other neuropathic disabilities, cardiac arrhythmia, precordial discomfort and intractable cough. Although rare, the occurrence of vaccine associated deleterious effects has been well-known and often over-reported. In contrast, the beneficial side effects were rarely, if ever, mentioned partly because they were so rare as to go unnoticed by the patients or their physicians, or partly because their reports were rejected in disbelief by the editors of medical journals. These include relief of pain, palsy and cough, and the uncovering of an occult chronic intestinal infection leading to its definitive treatment and recovery. Of greater importance is the possibly favourable influence of COVID-19 vaccine in cancer. All cancer patients in this study did unusually well after vaccination, in spite of being sometimes at a rather advanced or high risk status of cancer development. The possibilities of beneficial effects from vaccines are further discussed in the respective section. This study was made against a back ground of the Hong Kong Special Administrative Region, China, with a population of over seven million, of which over 90% had received at least two doses of COVID-19 vaccine. This is a candid report of the off-target effects of vaccination, significant enough to cause the patient to report to a hospital clinic, including the good, the bad and the possibly beneficial, presented with comments on their characteristics, their possible mitigation and even their potential therapeutic value.
Keywords
COVID-19 vaccines; Fatigue; Infection; Myocarditis; Virus
Introduction
For three years, the COVID-19 pandemic has been ravaging the whole world with unprecedented magnitude. For two years, specific COVID-19 vaccines have been developed with record speed and efficiency. Yet, to this day, large numbers of the world’s population remain unvaccinated and, regrettably, the anti- vaccination sentiment has remained high in some sectors [1,2]. This problem substantially contributed to the present situation where the causative virus, SARS-CoV-2 remains largely out of controlled, and most, if not all countries are resigned to a policy of co-existence with the virus. Much of the anti-vaccine sentiment stems from worries of the vaccine side-effects [2]. Such worries tend to be magnified by misrepresentation and exaggeration. On the other hand, under-reporting and even cover-up of vaccine side- effects raise more suspicions. Selective and biased reporting to serve monetary or political purposes has often been the prerogative of the media. All these add up to the public’s mistrust of the vaccine drive. Furthermore, almost all reports were confined to deleterious side effects without mentioning any side effects that might prove otherwise [3]. This did little to promote vaccine uptakes. The present study describes the real-world findings in a front-line clinical setting on the vaccine side-effects, including both the good and the bad, hoping that credibility and mutual trust may be restored among the vaccine hesitators or even the opponents. Due to the small sample size this is by no means close to a comprehensive review, but it carries the strength of a real-life clinical bedside approach rather than being buried in a mountain of fact sheets and statistical analysis. Instead of rounding up by just parochially repeating how rare and insignificant are the side-effects or how favourable is the risk/benefit ratio, practical and positive methods are boldly proposed to try to understand these problems and mitigate them if possible. Hopefully, this approach might help to dispel further fear or misunderstanding.
Materials and Methods
The two available COVID-19 vaccines available in Hong Kong are the mRNA-based Comirnaty, originally designated BNT 162b2, produced by BioNTech, Germany, (hereafter called Comirnaty), and the inactivated virus-based vaccine, CoronaVac, produced by Sinovac Life Science, Beijing, (hereafter called Sinovac) [4]. From mid-2021 to the end of 2022, there were 15 patients who presented to a hospital-based clinic with significant or serious clinical events related to the side effects of the two COVID-19 vaccines (Table 1).
There were eight male patients to seven female patients giving an even distribution between male and female sex. The age ranged from 23 to 101 years. Comirnaty was given to twelve patients and Sinovac to four, with one patient receiving both vaccines sequentially. Unexpectedly, four beneficial effects were seen, three with Comirnaty and one with Sinovac. Another five patients might have derived some benefit from vaccination in their anti-cancer management. Two further cancer patients had their low-grade breast cancer resected over ten years ago with no evidence of recurrence. The intriguing and complex relation between COVID-19 vaccines and cancer will be dealt with in a subsequent dedicated section. As expected, some deleterious effects were observed totalling 22 events, 19 with Comirnaty and three with Sinovac. Usually, more than one side effect occurred in a patient, and up to six significant side effects were observed in Cases No. 8 and No. 13. These side-effects are further elaborated individually in the following sections.
Pain
Pain is naturally the commonest unpleasant side-effect of any vaccine by injection [5]. It could vary greatly from person to person and, for the same person, from one injection to the next. To allay the patient’s worries, it is important to understand and explain the varying features of pain, as illustrated in the following five cases.
One patient (Patient No. 8) had it so severely that for weeks he could not allow his affected right shoulder to be touched, nor could he move or make use of his right arm. It turned out that he was having concurrent damage to the right brachial plexus which lingered long after the pain had subsided. Another patient (Patient No. 2) gave the intriguing story that initially he had all three Comirnaty injections on the left deltoid muscle. Each time the pain in the injection site grew more severe. After the third dose, the pain was not only severe but also persistent, lasting six months, when he received the booster fourth dose. He decided to change the injection site to the opposite shoulder. To his pleasant surprise, the pain in both shoulders subsided within a few days. Another person (Patient No. 10), after receiving the same vaccine, had very minimal pain on the left shoulder, but the pain increased in severity with each succeeding injection. Then he took the booster 4th injection on the opposite shoulder. Instead of subsiding, the pain in the original site flared up as well, apparently following an anamnestic reaction, and he ended up with moderate amount of shoulder pain on both sides for a few days.
In Hong Kong, we always advise all those who had three injections of the inactivated virus vaccine, Sinovac, to take the mRNA vaccine, Comirnaty, for a booster 4th dose because the latter elicits a higher level of antibodies [1]. As a second injection tends to be more painful than the first, this gives rise to the impression that mixed vaccination is more painful or that Comirnaty vaccine is more painful. But we have seen a case of negligible pain with Comirnaty as the first injection followed by severe pain with Sinovac for the second injection (Patient No. 13). The truth probably is that booster doses sometimes tend to be more painful regardless of vaccine types (Table 2).
Diarrhoea and fever
Three patients had diarrhoea after receiving the mRNA Comirnaty vaccine. One (Patient No. 8) was a 23 year-old male lawyer with diarrhoea up to over 10 times a day and temperature up to 38.3°C. Another (Patient No. 9) was a 101 year-old lady with diarrhoea up to three times a day and temperature up to 38.0°C. Both patients were known to have their normal temperatures between 36.0 to 36.5°C and the rise in temperature was considered significant. In the case of the young man it turned out that he had mild bowel upset since childhood due to occult double infection with Campylobacter and Cryptosporidium. The vaccination probably tipped the balance of the gut microbiome further, resulting in severe intractable diarrhoea. Control of the diarrhoea got rid of his chronic bowel problems. As both cases had been separately reported earlier their details will not be repeated here [6,7].
A third patient (Patient No. 13), a 53 year-old lady had watery diarrhoea twice, but no fever. As her symptoms were dominated by palpitation and cardiac arrhythmia, she will be further discussed in detail in the section of cardiac side-effects (Table 3).
Fatigue as a major symptom
Some degree of fatigue often accompanies other side effects especially with diarrhoea or severe pain. Occasionally fatigue could be the major presenting symptom. A 66 year-old female (Patient No. 6) with well controlled type 2 diabetes mellitus, suffered from severe fatigue after her second injection of Sinovac. As she was completely symptomless after her first injection of the same vaccine she was over-confident with the second injection. She took it in early morning and immediately went hiking with her pre-teen grandchildren. Under the blazing sun the children skipped and ran all over the hilly countryside, giving her a hard time to catch up with them. She was completely exhausted and had to rest at home for the next three days. Most people might feel some very mild fatigue after the second dose of vaccine or after a day of hiking and just ignore it, but her case was exceptionally severe, likely due to the combination of vaccination, strenuous exercise, and dehydration. The relation of exercise to excessive fatigue will be further discussed in the section on mitigation of side effects. Three more patients had marked fatigue, the young lawyer (Patient No. 8) with severe diarrhoea, neuralgia and neuropathy who took six months to fully recover, the centenarian (Patient No. 9) who also had diarrhoea and dementia but picked up after two days of parenteral fluid replacement, and a patient (No. 13) presenting also with cardiac arrhythmia, mild fever and diarrhoea. Fever, pain and fatigue form a common triad, often following the second injection of vaccines, either Comirnaty or Sinavac (Table 4).
Neurological complications of COVID-19 vaccines
Much has been written on the neurological complications of COVID-19 vaccines. The list includes Guillian-Barre syndrome, acute transverse myelitis, encephalitis, epileptic seizures, neuralgia, paraesthesia, anosmia, dysgeusia, the much-publicized cerebral venous thrombosis mainly related to adenovirus vector-mediated dsDNA vaccines, and facial palsy [8]. We encountered none of these entities except a case of right brachial plexus neuropathy (Parsonage-Turner syndrome) in patient No. 8 and another exceptional but interesting case of facial palsy recovery after taking the vaccine.
Brachial plexus neuropathy (Parsonage-Turner Syndrome)
The same patient (from Table 1, patient no. 8) who developed severe shoulder pain and diarrhoea continued to have weakness of the right upper limb. In fact, the weakness grew more obvious two weeks after the Comirnaty injection when the diarrhoea came under control. Although initially the weakness was considered due to severe pain in the muscle injection site, at the end of three months the pain had cleared up but the weakness persisted. At this point, he had a detailed assessment by a neurologist who confirmed the brachial plexus neurological damage being consistent with Parsonage-Turner syndrome. He was given a supplement of Vitamin B complex, reinforced with B12, but no corticosteroids. By the end of 6 months he made a complete recovery. He declined any second vaccination but remained free from any COVID-19 infection. (N.B. This patient’s predominant acute presentation was severe, protracted diarrhoea and had been separately reported in that context) [6].
Parsonage-Turner syndrome is known to result from direct trauma, viral or bacterial infection, autoimmune disorders, and vaccination including the Covishield vaccine by AstraZenaca, the mRNA-1237 by Moderna, and the BNT162b/Comirnaty by BioNTec [9-11].
Facial nerve palsy resolution
Facial nerve palsy is a much publicized but not entirely substantiated complication of both COVID-19 infection and its vaccines. Reports from Hong Kong suggest a slight and insignificant increase with Sinovac but no increase with Comirnaty vaccine [12]. We have not seen any case of facial nerve palsy in this small study. Surprisingly, we do see a case of longstanding facial nerve palsy of nine years dramatically improved after the first injection of Comirnaty and almost completely resolved after the second injection. He proceeded to take the third dose of mRNA vaccine uneventfully. Again, as this case is already reported previously, the reader is referred to the online free access journal for further details [13].
COVID-19 vaccination among cancer patients
An extensive study in China found that the Sinovac COVID-19 vaccine might enhance the efficacy of immunotherapy of nasopharyngeal carcinoma but not several other cancer types [14]. Among matched metastatic nasopharyngeal cancer patients receiving combined immunotherapy and chemotherapy, the observed response rate was 59.0% for the vaccinated group versus 35.7% for the unvaccinated group. We do not have patients undergoing cancer immunotherapy in this study, but we do have seven cancer patients in this group having received 1 to 4 shots of vaccine (Table 5).
Judging by usual standards, the first five patients on the table should be considered high risk cases. Patients No. 1 and No. 5 were stage 4 cancers with successful removal of their primary tumour as well as their respective oligo-metastases. Patient No. 2 had relatively early cancer, pathological classification T3N0. Yet, he had end stage diabetic nephropathy sustained by a renal transplant for the past 15 years and was committed to life-long immune suppression, a serious risk for cancer recurrence and progression. Patient No. 3 was a locally advanced squamous cell lung cancer, bordering on being inoperable from first diagnosis. A delay due to COVID-19 infection led to further advancement of the cancer. Surgically, he was considered inoperable. Yet, after five rounds of anti-cancer chemotherapy, with three doses of Comirnaty sandwiched in between, he had his lung cancer down-staged from Stage IIIc to Stage IIIa and surgical resection became feasible. Unfortunately, at this stage he caught a second COVID-19 infection, ten months after the first infection. Although this “break-through infection” was so mild as to be almost asymptomatic, it would be prudent to further postpone his surgery, as any major operation within eight weeks of a COVID-19 infection carries a substantially higher mortality [15].
In spite of their unfavourable factors, the first five patients did remarkably well. Patients No. 1, 2, 5 had all their detectable cancers resected. Follow up from nine to eighteen months showed no sign of recurrence. Even patient No. 3 showed remarkable regression of the size and activity of the cancer so that we are considering its resection once again – as an example of successful down-staging of the cancer with neoadjuvant chemotherapy. Patient No. 11 had poorly differentiated adenocarcinoma with metastasis to five of six para-aortic nodes. Remarkably all the hilar nodes were microscopically cancer-free, and all the deposits in the para-aortic nodes were rather scanty with cancer cells as if being subdued by some anti-cancer factors (but no neoadjuvant therapy was given), and she did well after surgery.
The last two patients were considered low risk breast cancers and were expected to do well. Patient No. 15 had a ductal carcinoma in situ with extensive involvement of both the upper half and the lower lateral quadrant of the left breast. There were signs of malignancy in the MRI and blood-stained nipple discharge. Left mastectomy with block dissection of axillary tissue was carried out fifteen years ago. Histology showed papillary architecture, ER+ve, PR+ve, HER2-ve, Ki index 4% (low). Since then she did well with no sign of recurrence. When COVID-19 swept over Hong Kong she had already retired and lived in a nursing home. All 90 occupants of the nursing home had four injections of Sinovac, but all came down with breakthrough infection except her. While the 15 year long gap between removal of her breast and the COVID-19 pandemic suggested no relation between them, her exceptionally good defence against the virus raised the spectre that past victory over cancer might somehow correlate with higher chance of success against COVID-19 with the help of vaccines. However, patient 13 also had her breast cancer removed over ten years and yet had a breakthrough COVID-19 infection in spite of three vaccine injections. She will be further discussed in the section on cardiac arrhythmia (Table 5).
Based on such unusually favourable outcomes, it is tempting to speculate that the interaction between COVID-19 vaccine and cancer, with or without the addition of COVID-19 infection, might somehow contribute to a more favourable outcome. This section is only a broad preliminary overview of the intricate interaction between COVID vaccination and certain cancers. A more detailed analysis of the subject will follow after further studies in the near future.
Intractable cough (From Table 1. Patient No. 4)
Cough, often dry and distressful, is one of the commonest symptoms of COVID-19, and is also listed as an allergic side effect of COVID-19 vaccines [16]. We have a 72 year-old lady with good past health who received the second dose of inactivated virus vaccine Sinovac in May, 2022, and almost immediately developed a dry distressful cough. The cough was resistant or had very short-lived response to all the medications tried on her including anti-histamines, mucolytics and expectorants, codeine derivatives, and a rotation of antibiotics ranging from ciprofloxacin through macrolides and cephalosporins to tetracyclines. Her tuberculin skin test was negative. Sputum culture was negative for pathogenic bacteria, acid fast bacilli, and fungus. Multiplex panel for respiratory tract pathogens was negative to all 31 bacterial, viral, fungal and parasitic pathogens. The remote possibility of Loeffler’s syndrome was considered without further evidence of parasitic infection [17]. A blind trial of anti-parasitic agent (Mebendazole) yielded partial, short-lived relief.
At the end of six months she had the booster third dose of Sinovac and almost immediately the cough subsided. This case is reminiscent of the patient who developed severe persistent L shoulder pain after the third dose of Comirnaty but was relieved after the fourth dose. The mechanism underlying such intriguing symptom-relief upon re-vaccination awaits further research.
Results and Discussion
It is well-known that vaccines take one to two weeks to induce effective antibodies and virus-specific T-cells. For this reason, almost all studies excluded subjects who were less than two weeks after the injection. But in real-life the virus may not give humans that chance of two-week break. During the onslaught of a new wave of the pandemic, people were awakened to the urgent need of vaccination and flocked to the vaccination centres. Some of these vaccine-seekers might be already infected and spread the virus to others in the vaccination centre. Very little study was made on this practical issue. We advocate screening all intended vaccine recipients with the rapid antigen test (RAT), but that is not the practice in vaccination centres. In any case RAT might not pick up all the carriers. Even screening with the RT-RNA PCR test does not necessarily pick up every COVID-19 carrier in their pre-symptomatic stage, nor is it practical to carry out such tests in a busy vaccination centre.
Patient No. 12 illustrates this problem. He and his wife each had two doses of Comirnaty vaccine in August and September 2021. During March 2022 they were due for the booster third dose but the fifth wave of the pandemic was reaching its peak in Hong Kong. The husband went to the vaccination centre for his third dose of Comirnaty, while the wife, being a patient with life-long bronchiectasis, discreetly stayed at home. On the same morning, an asymptomatic carrier also attended the same vaccination centre and passed the infection to him. He returned home and passed the infection to his wife. The wife now had a natural booster dose of live virus. She suffered minimal symptoms and made a speedy recovery. But the husband infected with the same virus had severe symptoms of fever and dry cough which dragged on for six months.
It is tempting to speculate that the vaccine given on the same day as the infection might be a possible cause of the severe symptoms. Indeed, during the development of antiviral vaccines one of the principal concerns was the phenomenon of Antibody Dependent Enhancement (ADE) for the virus infection [18]. The antibody induced by the vaccine on the first day might not only fail to neutralize the virus, but actually enhance virus entry into host cells and enhance the virus infection. Alternatively, the antigen stimulation from the newly (but untimely) injected booster third dose of vaccine might have jeopardized the natural production of effective antibodies by the existing immune system. Other mechanisms might be at work and it will take more research to find out. In any case, it would be prudent to avoid getting infected and vaccinated at the same time.
Cardiac side effects (Table 1. Patient Reference No. 13)
Myocarditis pericarditis and postural orthostatic tachyarrhytthmia are well known and widely reported complications of COVID-19 vaccines, but they are usually very rare, and not encountered in this small study [5,19,20]. We do find that palpitation and chest discomfort are not uncommon but overlooked most of the time. Even when asked with leading questions most vaccine recipients will gloss over such symptoms as being too trivial to mention so that the true incidence remains obscure.
We do have a patient who developed palpitation and chest pain severe enough to result in hospitalization (Patient No. 13, Tables 1-4). This was a 53 year-old lady who had a small breast cancer in situ excised 10 years ago with no sign of recurrence. On the day after receiving the first injection of Comirnaty vaccine, she developed marked palpitation, chest discomfort and precordial pain. She did not notice any significant pain in the injection site (which was probably overshadowed by the alarming cardiac symptoms). She was admitted into hospital and put on continuous telemetry ECG monitoring.
Initially, her ECG showed a basic rhythm of sinus bradycardia at 50 per minute, with frequent ventricular extra-systoles which brought on chest pain whenever it occurred. T-wave was inverted in V1 and V2 and flattened in other leads.
Subsequently three additional abnormalities appeared, independent of each other: (1) short runs of ventricular tachycardia or ventricular fibrillation, lasting for a few seconds but non-sustained from figure 1, (2) change of T-wave from upright to inversion in Lead 2 lasting two days, (3) deep Q-wave in Lead 1 which lasted for another two days. Each additional ECG abnormality aggravated her symptoms of precordial pain and discomfort.
After seven days her ECG settled to a sinus rate of 52 to 60 with occasional ventricular extra-systoles, and she was discharged on condition of high alert and low threshold of readmission.
Her cardiac enzymes including Troponin I, NT-proBNP and D-dimer remained normal throughout her stay in hospital. Her echocardiogram was normal on both admission and discharge. Her cardiac MRI showed early gadolinium enhancement consistent with capillary leak in early myocarditis. But as there was no late gadolinium enhancement, myocardial injury could not be confirmed. The diagnosis remained as “cardiac arrhythmia” and not “myocarditis”.
The next problem was the choice of the second dose of vaccine. After consulting a cardiologist and a specialist in allergy and immunology we finally settled on the choice of the Sinovac vaccine. This was administered exactly eight months after the first dose of Comirnaty, and to our relief, she had no cardiac problems. However, she developed a different set of reaction with fever, fatigue and considerable pain around the injection site. She also had considerable anxiety over the efficacy of the Sinovac against the new Omicron virus mutants. Test for antibody titre was suggested but declined by the patient. Instead, she proceeded with a second dose of Sinovac and experienced the unpleasant reaction of fever, fatigue and pain in the site of injection. Four months later, she had the COVID-19 infection, which turned out to be very mild (Figure 1).
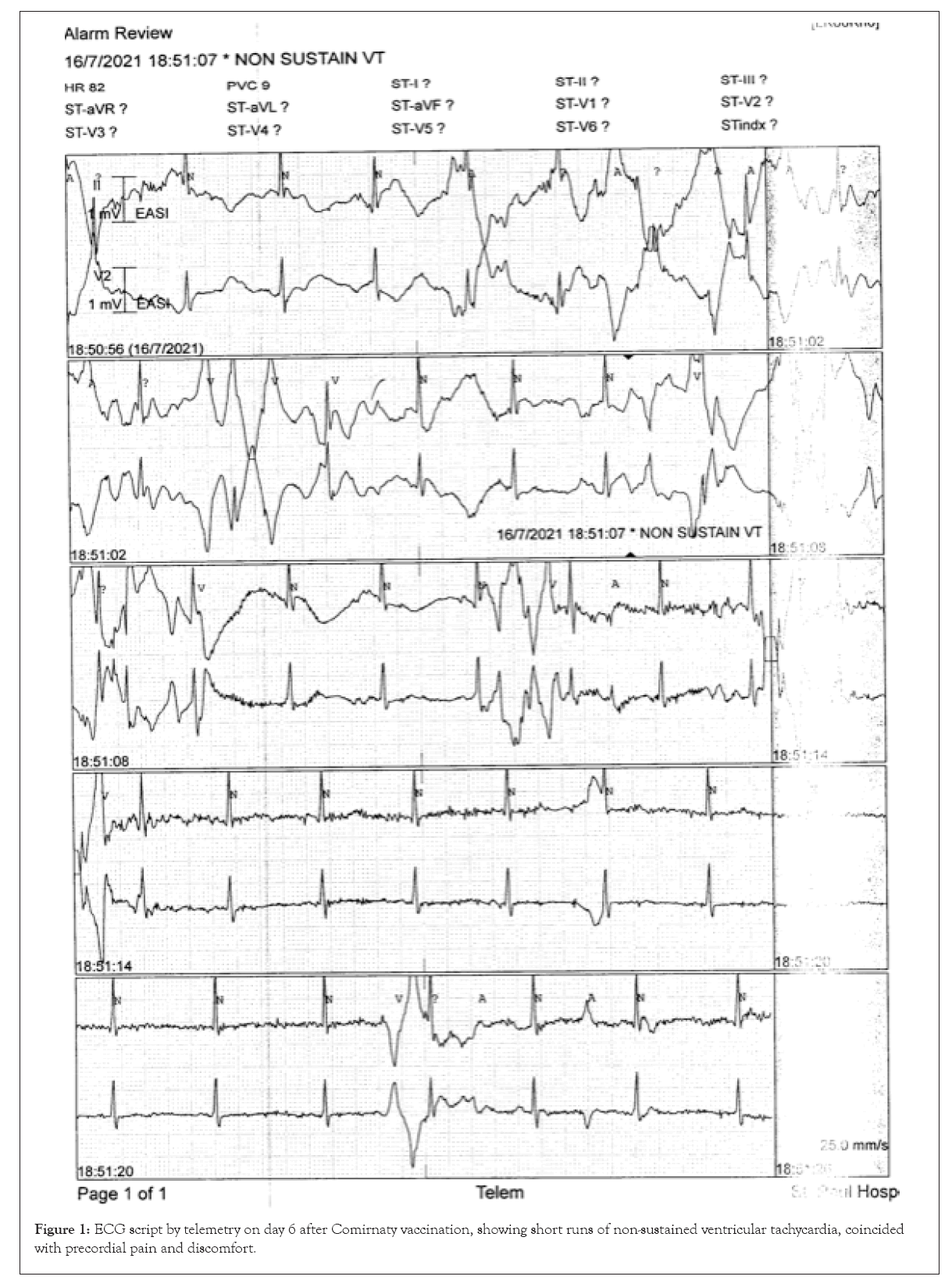
Figure 1: ECG script by telemetry on day 6 after Comirnaty vaccination, showing short runs of non-sustained ventricular tachycardia, coincided with precordial pain and discomfort.
Immune disorders and vaccination
Intuitively one would expect patients with immune disorders to develop some increased risk of disease reactivation after vaccination, but it did not happen in this study. We have seen a case of systemic lupus in remission going through three Comirnaty injections uneventfully. We have also seen a 71 year-old patient with recent severe urticaria due to zinc allergy who went through four Comirnaty injections without any sign of allergy flare-up. A 64 year-old lady had familial anti-phospholipid syndrome with thrombophilia and recurrent deep vein thrombosis in both legs. She took her first dose of inactivated virus Sinovac and had dinner with an asymptomatic carrier the same evening. Next day, she was tested COVID-19 positive. Yet, she had no particular adverse symptom or flare up of her disorder. This case stood in stark contrast with patient No. 12 in Table 1 who suffered badly from synchronous COVID infection and COVID vaccination on the same day in the same vaccination centre. It seems that pre-existing autoimmune disorders does not predict increased COVID-19 severity nor undesirable vaccine side-effects. If anything, her on-going long-term corticosteroid and anticoagulant therapy might have mitigated any COVID-19 infection and vaccine reactions. As all patients discussed in this paragraph had no side effects from the vaccines, they were not included in the 15 cases in Table 1.
| Patient Reference No. | Sex | Age | Vaccine | Side effects | Remarks |
|---|---|---|---|---|---|
| 1 | M | 71 | Comirnaty | Possibly contributed to favourable outcome of cancer treatment | Possibly beneficial |
| 2 | M | 66 | Comirnaty | Relief of previous persistent injection site pain with 4th dose | Beneficial |
| Possibly contributed to favourable outcome of cancer treatment | Possibly beneficial | ||||
| 3 | M | 74 | Comirnaty | Possibly contributed to favourable outcome of cancer treatment | Possibly beneficial |
| 4 | F | 72 | Sinovac | Severe refractory cough × 6 mths after 2nd dose of vaccine | Deleterious |
| Relief of refractory cough after 3rd dose of vaccine | Beneficial | ||||
| 5 | M | 68 | Comirnaty | Possibly contributed to favourable outcome of cancer treatment | Possibly beneficial |
| 6 | F | 66 | Sinovac | Severe fatigue after 2nd dose of vaccine | Deleterious |
| 7 | M | 49 | Comirnaty | Recovery from facial palsy of 9 years standing | Beneficial |
| 8 | M | 23 | Comirnaty | Severe disabling pain R shoulder × 3 mths | Deleterious |
| Brachial Neuropathy (Parsonage-Turner syndrome) | Deleterious | ||||
| Severe diarrhoea × 2 weeks, dehydration | Deleterious | ||||
| Fatigue, fever | Deleterious | ||||
| Revelation of occult intestinal infections leading to eradication | Beneficial | ||||
| 9 | F | 101 | Comirnaty × 3 | Fatigue | Deleterious |
| Diarrhoea, fever | Deleterious | ||||
| 10 | F | 59 | Comirnaty | Possible contribution to anti-cancer treatment | Possibly beneficial |
| 11 | M | 87 | Comirnaty | Recall pain in previous vaccine injection sites | Deleterious |
| 12 | M | 66 | Comirnaty | Enhanced severity of COVID-19 contracted on same day as 3rd dose | Deleterious |
| 13 | F | 53 | 1st dose Comirnaty | Marked cardiac arrhythmia and precordial pain × 1 week | Deleterious |
| Fatigue | Deleterious | ||||
| Diarrhoea and fever | Deleterious | ||||
| Sinovac × 2 | Severe pain at injection site | Deleterious | |||
| COVID-19 breakthrough infection 4 months after last Sinovac | |||||
| 14 | F | 64 | Comirnaty | Generalized myalgia and arthralgia increasing from2nd to 4th dose | Deleterious |
| 15 | F | 95 | Sinovac × 4 doses | Post mastectomy 15y, no sign of recurrence, remain the only one uninfected by COVID-19 among 90 residents in an elderlies’ home | Cancer too remote in the past |
Note: All patients were de-identified. These reference numbers are solely for presentation of this study and bear no clue to their actual hospital numbers. Age refers to the age at first vaccination.
Table 1: List of patients experiencing significant side effects with COVID-19 vaccines
| Patient Ref. No.* | Sex | Age | Vaccine type | Nature (injection site de facto) | Management | Outcome |
|---|---|---|---|---|---|---|
| 2 | M | 66 | Comirnaty 4 doses | Increased severity up to 3rd dose | Observe | Resolved with 4th dose |
| 8 | M | 23 | Comirnaty 1 dose | Severe disabling pain × 3 months | Analgesics, rest | Resolved after 3 months |
| 11 | M | 87 | Comirnaty 4 doses | Increased severity with each dose, recall pain in previous sites | Observe | Resolved |
| 13 | F | 53 | Comirnaty 1st dose, Sinovac 2nd dose | 1st dose negligible pain; mainly chest pain and cardiac arrhythmia | Analgesics | Resolved |
| 2nd dose severe pain injection site | ||||||
| 14 | F | 64 | Comirnaty 4 doses | Generalized myalgia and arthralgia increasing from2nd to 4th dose | Deleterious | Resolved |
Note: *These reference numbers correspond to the same patients in Table 1.
Table 2: Side effects of COVID-19 vaccination-Pain, significant to severe
| Patient Ref. No. | Sex | Age | Vaccine type | T °C | Nature (injection site de facto) | Management | Outcome |
|---|---|---|---|---|---|---|---|
| 8 | M | 23 | Comirnaty 1 dose | 38.3 | Watery stool 2-10 × daily × 2wks, dehydrated, required i.v. drip | Double rare infection found | Cured by appropriate antibiotics |
| 9 | F | 101 | Comirnaty 1st,2nd | 38 | Watery stool 3 ×/day × 2 days, also fatigue and mild fever | i.v.drip | Resolved |
| 13 | F | 53 | Comirnaty 1st dose | 37 | Watery stool 2 ×/day × 2 days, also cardiac arrhythmia and fever | Observe | Resolved |
Note: *These reference numbers correspond to the same patients in Table 1.
Table 3: Side effects of COVID-19 vaccination-diarrhoea
| Patient Ref. No.* | Sex | Age | Vaccine type | T °C | Accompanying disorder | Management | Outcome |
|---|---|---|---|---|---|---|---|
| 6 | F | 66 | Sinovac 2nd dose | 37 | DM2 (controlled), over-exertion | Home rest | Resolved after 3 days |
| 8 | M | 23 | Comirnaty 1 dose | 38.3 | Diarrhoea, severe shoulder pain | Hospitalization, i.v. drip | Recovered after 6 mth |
| 9 | F | 101 | Comirnaty 1st, 2nd | 38 | Diarrhoea, fever | i.v.drip | Resolved |
| 13 | F | 53 | Comirnaty 1st dose | 37 | Cardiac arrhythmia, diarrhoea | Hospitalization, i.v. drip | Resolved in 2 weeks |
Note: *These reference numbers correspond to the same patients in Table 1, DM2, type 2 diabetes mellitus.
Table 4: Side effects of COVID-19 vaccination-fatigue
| Patient Ref. No.* | Sex | Age | Diagnosis | Co-morbidities | COVID vaccines | COVID infection | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | M | 71 | Colon Ca op. 8yr. metastasis RUL | Comirnaty × 1 | No | Right upper lobectomy for lung metastasis | |
| 2 | M | 66 | Adenocarcinoma lung T3N0 | DM2, Renal transplant 15y, on immune suppression Rx | Comirnaty × 4 | No | Apicosegmentectomy left lower lobe |
| Well × 8m | |||||||
| 3 | M | 74 | SqCLC stage IIIc | DM2,CAD, stent LAD × 17y, LCX moderate dis. | Comirnaty × 3 | Yes × 1 before and 1 after vaccines | Chemo down-staged Ca to IIIa, considering surgery or radiotherapy |
| 5 | M | 68 | SqCLC, metastasis to L femur shaft and L buttock | DM2 | Comirnaty × 3 | Yes × 1, after 3 vaccine doses | L pneumonectomy, resect femur section and buttock mass, RT, 18m |
| 11 | F | 59 | Adenocarcinoma T3N2 | Mental depression | Comirnaty × 2 | Yes × 1, after 2 vaccine doses | L upper lobectomy + radical mediastinal dissection |
| 15 | F | 95 | Diffuse DCIS with papillary structure in left breast | Sinovac × 4 | No | Had L mastectomy 15 years ago; only resident in nursing home remained uninfected | |
| 13 | F | 53 | Localized DCIS, lumpectomy 10 yr ago | 1st dose Comirnaty | No | Marked multiple cardiac arrhythmia, diarrhoea | |
| 2nd and 3rd doses Sinovac | Yes, 4m after last Sinovac | Fever, severe pain at injection site |
Note: *These reference numbers correspond to the same patients in Table 1.
Ca: Cancer; CAD: Coronary Artery Disease; DCIS: Ductal Carcinoma In Situ; DM2: Diabetes Mellitus Type 2; L: left; LAD: Left Anterior Descending Coronary Artery; LCX: Left Circumflex Coronary Artery; m: Month; RT: Radiotherapy; Rx: Therapy; SqCLC: Squamous Cell Lung Cancer; y: Year.
Table 5: Cancer patients and COVID-19 vaccines
| Patient Reference No. | Sex | Age | Vaccine | Beneficial off target effects |
|---|---|---|---|---|
| 2 | M | 66 | Comirnaty | Relief of previous persistent injection site pain with a 4th dose |
| 4 | F | 72 | Sinovac | Relief of refractory cough (following the2nd dose Sinovac) with a 3rd dose of Sinovac |
| 7 | M | 49 | Comirnaty | Progressive recovery from facial palsy of 9 years standing, from 1st through2nd dose |
| 8 | M | 23 | Comirnaty | Revelation of occult intestinal infections leading to their eradication with 1st dose |
Table 6: Patients who derived beneficial side effects after COVID-19 vaccination
Collective overview of beneficial vaccine side-effects
Altogether four patients seemed to derived some benefits after receiving COVID-19 vaccines. They were embedded in Table 1, amidst other cases and other more common but deleterious side effects. For clarity they are separately listed in Table 6. Patients No. 2 and No. 4 had the benefit of clearing up the deleterious side effects of the same vaccine they received previously, namely persistent pain in the previous Comirnaty injection site and persistent cough after a second dose of Sinovac. However, it was in the last two cases that the benefit was most dramatic. Case No. 7 was a businessman, who had to negotiate contracts with his clients [13]. He suffered from Bell’s palsy for nine years which persisted with only partial improvement after initial corticosteroid and antiviral treatment. The impediment considerably compromised his career prospects and after nine years he was resigned to his fate. After the first dose of Comirnaty his facial palsy made a 70% recovery which improved further to 80-90% recovery after the second dose and the gain was held steadfastly following the third dose. Six months later, his improvement was well maintained. The benefit with patient No. 8 was equally dramatic [6]. The patient was noted for his frail built which was blamed on his weak digestive system. The severe diarrhoea after his first dose of Comirnaty led to intense investigation and eradication of his intestinal pathogens and for the first time since his adolescence, he started to gain 10% in body weight bringing his BMI from 17.73 Kg/M2 to 19.14 Kg/M2. As mentioned in previous sections both Case No. 7 and No. 8 had been reported in detail before in the context of individual case reports but mentioned here for completeness of the present presentation (Table 6) [6,13].
Proposed methods of mitigation
Avoid stress, severe exertions and dehydration: The foregoing observations show that stress, severe exertion and dehydration should be avoided especially with second and subsequent vaccinations. Patients should be warned against strenuous exercise, drink plenty of water (perhaps avoiding artificially sweetened juice) and have adequate sleep. In severe cases with dehydration it may be necessary to give parenteral fluid replacement. There is a trend for health services to ask healthcare workers to carry on their demanding duties even when they do not feel well after COVID-19 infection or vaccination. Such demands are not only unfair and unreasonable, but may end up detrimental to the health service itself. If the staff collapses under working pressure, the medical service will end up with further shortage of manpower.
Avoid synchronous vaccination and COVID-19 infection: Receiving a dose of live virus infection and a shot of vaccine at the same time appeared to magnify the symptoms and delay the recovery. Both the health authority and the public should realize that the best time to vaccinate the population is before and not during the peak of the pandemic. With modern information technology, we can usually have some advanced warning of the advent of a new wave of infection. The public should be educated and arranged to receive the vaccine or its booster dose in advance.
Fractionated administration of vaccine in cases of potential severe reaction: We have redirected the patient with distressing cardiac arrhythmia after her first dose of Comirnaty to take the Sinovac for the second dose of vaccine. This may not be applicable in every situation. The patient may still develop a severe reaction to Sinovac, or Sinovac may not be highly effective against the newer omicron variant. Another alternative to mitigate this problem, successfully tested in Europe, is to re-administer the vaccine in fractionated form under cover of antihistamine and in a hospital setting [21].
Judicious choice of vaccines: In Hong Kong, we have not used other vaccines than the two described in foregoing sections. We have taken note of the increase in venous thrombosis especially intracranial venous sinus thrombosis following certain adenovirus mediated vaccines and chose to avoid them. Even with the limited choice of two types we still have to exercise great caution in the choice between them [22]. In general, the more potent Comirnaty vaccine is preferred over the Sinovac. However, if serious side effects were encountered with one we have to try the other in subsequent booster doses. In terms of efficiency, we advise those who were vaccinated with Sinovac to have at least one extra booster dose of Comirnaty in view of the latter’s superior protection against the SARS-COV-2’s omicron variant. Yet, we have not ruled out the possibility that the reverse selection of these two vaccines might work for some other virus variants in the future.
Judicious choice of methods of administering the vaccines: Theoretically, different methods of administering a vaccine may affect its efficacy and toxicity. In general, a smaller dose would elicit fewer side effects but, below a certain threshold, result in less protect. Unfortunately, the system of vaccine supply is such that the clinician is rigidly bound to follow the predetermine protocols and not permitted to make any adjustment at the individual levels – no room for personalized medicine. There are isolated examples in certain countries where discretion had been exercised by the managing team to overcome such problems as allergy and anaphylaxis [21]. How could we reconcile clinical versatility with public health dogmatism is beyond the scope of this study.
One of the most important and effective ways to control an epidemic is vaccination. The world has witnessed spectacular success in the past in our fight against many major and lethal infections, such as small pox, diphtheria, cholera, enteric fever, polio, just to name a few. In the present COVID-19 pandemic we have been able to work out the SARS-CoV-2 virus’ RNA sequence at record speed, followed by the production of effective vaccine at unprecedented efficiency [23,24]. Unfortunately, the implementation of universal vaccination to provide protection for mankind, and root out the virus at an early stage, met with vaccine hesitancy, scepticism and even stiff anti-vaccine sentiment which was backed by much misinformation. Consequently, we had missed the chance to stamp out the virus at an early stage but rather given it enclaves of human reservoirs to breed and mutate, providing the virus with the opportunity to develop wave after wave of new mutations capable of evading our immune mechanisms and re-infecting us with renewed vigour [14].
Conclusion
Contrary to the allegations of vaccine opponents and the misinformation they attempt to circulate, not all vaccine side effects are deleterious. In fact, some side effects might be even beneficial. Of particular interest is the interaction between vaccination and the immune system which may directly or indirectly influence the course of other aspects of health and disease. In particular, there are already certain signals that vaccination may enhance the efficacy of immunotherapy of cancer. It will take much more research to harness such interactions and apply them in therapeutics.
Limitations
This is a small clinical study with no big data, no impressive statistical analysis, no authoritative formulation of concepts and guidelines. The world’s leading scientific journals are already loaded with such articles. Rather, a different approach is taken, with first hand clinical material taken from real-life daily clinical practice of a front-line clinical worker. It carries the usual limitations of small sample size, single centre study and confinement to observations and annotations. Most of the observed features need further confirmation and their underlying biological and molecular mechanism will require further research to elucidate.
Acknowledgement
The author would like to thank all the patients in this study for their cooperation and for giving permission to use their clinical materials in this report.
Conflict of Interest
The author reports no conflict of interest in this report. No funding or financial assistance from any source was received throughout the study.
References
- The Government of Hong Kong Special Administrative Region. COVID-19 Vaccination Programme. Hong Kong Vaccination Dashboard. 2023.
- Dubé È, Ward JK, Verger P, MacDonald NE. Vaccine hesitancy, acceptance, and anti-vaccination: trends and future prospects for public health. Annu Rev Public Health. 2021;42(1):175-191.
[Crossref][Google Scholar] [PubMed]
- Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12):e2140364.
[Crossref][Google Scholar] [PubMed]
- The Government of the Hong Kong Special Administrative Region. “COVID-19 Vaccination Programme. About the vaccines. Possible side effects”. 2023.
- U.K. Government Medicines & Healthcare products Regulatory Agency – Patient information leaflet Comirnaty 30 micrograms/dose concentrate for age 12+ (purple cap)
- Leung JSM. Severe exacerbation of mixed Campylobacter and Cryptosporidium enteritis following BNT162b vaccination against COVID-19 – a vaccine and gut microbiota interaction that unveils a long-hidden disorder. Clinical Research and Clinical Trials.
[Crossref]
- Leung JS. COVID-19 in a centenarian, the vaccination, the breakthrough infection, and the third booster dose. Hong Kong Med J. 2023;29:181.
- Allahyari F, Molaee H, Hosseini Nejad J. Covid-19 vaccines and neurological complications: a systematic review. Z Naturforsch C J Biosci. 2023;78(1-2):1-8.
- Sharma A, Gupta A. A rare case of brachial plexus neuropraxia after COVID-19 vaccination. Cureus. 2022;14(1).
- Chua MM, Hayes MT, Cosgrove R. Parsonage-Turner syndrome following COVID-19 vaccination and review of the literature. Surg Neurol Int. 2022;13.
- Coffman JR, Randolph AC, Somerson JS. Parsonage-Turner syndrome after SARS-CoV-2 BNT162b2 vaccine: a case report. JBJS Case Connect. 2021;11(3):e21.
- Wan EY, Chui CS, Lai FT, Chan EW, Li X, Yan VK, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64-72.
- Leung JSM. Resolution of longstanding unilateral facial palsy after receiving BNT-162b vaccine: off target beneficial effect or reverse nocebo phenomenon?. J Clin Lab. 2022;5(5).
[Crossref]
- Hua YJ, Liu YL, Wen K, Kurts C, Wu H, Mei Q, et al. Potentially improved response of COVID-19 vaccinated nasopharyngeal cancer patients to combination therapy with anti-PD-1 blockade and chemotherapy. Ann Oncol. 2023;34(1):121-123.
- Deng JZ, Chan JS, Potter AL, Chen YW, Sandhu HS, Panda N, et al. The risk of postoperative complications after major elective surgery in active or resolved COVID-19 in the United States. Ann Surg. 2022;275(2):242.
- NHS inform/Services Scotland. Side effects of the coronavirus vaccines. 2023
- Kunst H, Mack D, Kon OM, Banerjee AK, Chiodini P, Grant A. Parasitic infections of the lung: a guide for the respiratory physician. Thorax. 2011;66(6):528-536.
- Huisman W, Martina BE, Rimmelzwaan GF, Gruters RA, Osterhaus AD. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27(4):505-512.
- Chua GT, Kwan MY, Chui CS, Smith RD, Cheung EC, Ma T, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2022;75(4):673-681.
- Kwan AC, Ebinger JE, Wei J, Le CN, Oft JR, Zabner R, et al. Apparent risks of postural orthostatic tachycardia syndrome diagnoses after COVID-19 vaccination and SARS-Cov-2 Infection. Nat Cardiovasc Med. 2022:1-8.
- Rivolta F, Camilla C, Sangalli A, Chiei Gallo A, Pravettoni V. Successful fractionated undiluted doses of COVID-19 vaccine in five cases of suspected allergic reactions to the first dose. Clin Case Rep. 2022;10(10):e6348.
- Andrews NJ, Stowe J, Ramsay ME, Miller E. Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: a national cohort study in England. Lancet Reg Health Eur. 2022;13:100260.
- Zhang YZ. Novel 2019 coronavirus genome: SARS-CoV-2 coronavirus. Virol. 2020.
- WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. 2022.
Author Info
John SM Leung*Citation: Leung JSM (2023) Clinical Examples of the Side-Effects of COVID-19 Vaccines, the Good, the Bad and the Unexpected: A Hong Kong Study. Appli Microbiol Open Access. 9.255.
Received: 20-Mar-2023, Manuscript No. AMOA-23-22283 ; Editor assigned: 22-Mar-2023, Pre QC No. AMOA-23-22283(PQ); Reviewed: 07-Apr-2023, QC No. AMOA-23-22283 ; Revised: 18-Apr-2023, Manuscript No. AMOA-23-22283(R); Published: 26-Apr-2023 , DOI: 10.35284/2471-9315.23.9.255
Copyright: © 2023 Leung JSM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.