
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research - (2022)Volume 5, Issue 11
Background: Hyper ferritinaemia and dysfunctions in iron metabolism are common presentations encountered in clinical practice. Iron overload relates to the metabolism of iron absorption, transport and storage and can lead to significant end organ dysfunction. Iron overload has multiple causes including Hereditary Hemochromatosis (HH), which is a heterogeneous group of genetically inherited disorders of iron metabolism. There are several gene mutations responsible for the development of HH, of which, mutations in the Homeostatic Iron Regulator (HFE) gene are the most common, accounting for approximately 80% of cases. Approximately 20% of patients with HH have mutations in non-HFE genes, including genes that express hemojuvelin, hepcidin, transferrin receptor 2 and ferroportin, each of which have important functions in iron metabolism. Some examples of non-genetic causes of hyper ferritinaemia include malignancies, infections, inflammatory disorders or iatrogenic causes (e.g. frequent blood transfusions or intravenous iron infusions), and can similarly contribute to clinical manifestations of iron overload. Hence, patients with non-HFE hyper ferritinaemia treated in community clinical setting includes both uncommon mutations (non-HFE HH) and other non-hereditary causes of hyper ferritinaemia. The recommended long term treatment strategies for these non-HFE patients are yet to be established.
Aim: This study aims to evaluate clinical outcomes of patients with non-HFE hyperferritinaemia treated in the community with lifestyle modifications and venesection.
Methods: In this one-group pre-test post-test pilot study, 120 patients with non-HFE hyper ferritinaemia were studied. All patients underwent laboratory investigations including serum ferritin/transferrin saturation, inflammatory and tumour markers, Liver Function Studies (LFT), Thyroid Function Studies (TFT), Blood Sugar Level (BSL) and CT scans. Patients were provided with lifestyle modification education; in cases of persistent hyper ferritinaemia (>6 months), venesection therapy was performed. The laboratory-based investigations were repeated after minimum of six months of therapy, and this data was compared to a control group. The statistical analysis was performed using the Wilcoxon test and McNemar test.
Results: Patients pre-treatment showed significantly higher serum ferritin levels compared with controls. 37 patients (31%) demonstrated an elevated ferritin level of ≥1000m mol/L. 74 patients had abnormal LFTs at baseline. While LFTs improved in 24 (32.4%) of these patients with lifestyle modifications, 36 (48.6%) required additional venesection therapy. 14 patients (19%) showed no improvement in LFTs even with addition of venesection therapy to lifestyle modification and successful reduction in serum ferritin. 38% of patients with a BMI >30 responded adequately to intervention, of which 63% required additional venesection therapy. Furthermore, amongst non-obese patients, 57% of patients required additional venesection therapy as lifestyle measures alone was inadequate.
Conclusion: Our study showed that there was a significant improvement in clinical and laboratory markers associated with end organ dysfunction in patients with non-HFE hyper ferritinaemia when treated with lifestyle modifications and venesection therapies.
Hyper ferritinaemia; Hemochromatosis; HFE gene
Hyper ferritinaemia is a common presentation encountered in clinical practice, and is characterized by increased serum ferritin levels reflecting an excess of iron storage or inflammatory states. Hyper ferritinaemia can be associated with iron overload, and diseases of iron metabolism. Hereditary Hemochromatosis (HH) is an inherited disease in which the absorption, transport and storage of iron exceeds requirements. It is characterized by an inappropriate increase in intestinal absorption of iron due to reduced expression or function of the iron regulatory protein, hepcidin. Untreated, it can progress to iron overload with various complications. [1-3]. Non-inherited causes of hyper ferritinaemia include infections, inflammatory disorders, malignancies, excess alcohol consumption, fatty liver, metabolic disorders, thalassaemia and transfusion related iron overload and others [4].
In normal iron metabolism, transferrin is produced in the liver and binds free iron. Its production increases in response to iron deficiency and will decrease in iron overload. It is a glycoprotein with two binding sites. The percentage of transferrin saturation will increase with iron concentration, normal being 30%, with 10% in the different state. On a daily basis, transferrin transports 20-40 mg of iron from stores to tissues. All cells accept iron from transferrin via cell membrane transferrin receptors. [5,6]. The Hepcidin-Ferroportin axis is a key regulator of iron homeostasis, and controls the absorption of iron from the small intestine, and mobilization iron stores from the liver, in response to inflammation or iron deficiency states. Ferritin is an intracellular protein that stores iron, primarily in the liver.
Ferritin molecules can aggregate to form insoluble hemosiderin, and this can occur in cases of iron overload. Importantly, from a clinical perspective, serum ferritin can be detected and, its amount is directly proportional to the amount of reticuloendothelial system macrophage stored iron, except in cases of inflammation or tissue damage [7]. Haemojuvelin is a membrane bound protein important in cell signaling that results in hepcidin production. Haemojuvelin deficiency leads to hepcidin deficiency, resulting in excess iron absorption from the small intestine [8,9].
HH is a heterogeneous group of inherited disorders that affect multiple genes in iron metabolism, all of which result in decreased Hepcidin. The Homeostatic Iron Regulator (HFE) gene is the most commonly affected gene, accounting for 80% of HH cases. Non-HFE genetic mutations occur in approximately 20% of patients with hereditary hemochromatosis, and are associated with dysfunctions in hemojuvelin, hepcidin, transferrin receptor 2 and ferroportin proteins.
There are five identified types of HH. Type 1, or HFE-HH, is an autosomal recessive condition with variable penetrance with more significant disease, commonly presenting in adulthood. [10] Types 2 to 5 HH are all caused by non-HFE genetic mutations. Type 2 HH the Juvenile variants present earlier and with more significant disease [11] and can include hypogonadism, diabetes, and cardiomyopathy [12]. It also follows an autosomal recessive inheritance pattern. Type 2A is due to mutations in the HJV gene (codes for haemojuvelin) and 2B is due to mutations in HAMP gene (codes for hepcidin). [13] Type 3 is due to mutations in the TFR2 gene, responsible for coding the transferrin-receptor 2. Disease severity in this type is moderate. [14] Type 4 is due to mutations in SLC40A1, coding the Ferroportin 1 gene. In contrast to types 1, 2, and 3, hepcidin levels are appropriately elevated, but without functioning receptors to influence; and it are inherited in an autosomal dominant pattern. [15] Type 5 is inherited in an autosomal dominant pattern and from a mutation in FTH1 [16].
A diagnosis of HH is suspected in patients with abnormal iron studies (elevated transferrin saturations and ferritin) with a family history of haemochromatosis. This diagnosis is confirmed by genetic testing, however, it should be noted that non-HFE causes of HH are not routinely investigated for on hemochromatosis gene panels, given they are relatively uncommon. Early diagnosis and prompt initiation of appropriate treatments are essential for preventing irreversible organ damage [17]. Hepatic function, glucose tolerance, cardiac function, thyroid function, and gonadal function should be evaluated once HH is diagnosed, and symptoms secondary to iron overload, such as diabetes, cardiomyopathy, hypothyroidism, and hypogonadism, should be appropriately treated by consultation with the specialists of these disorders [18]. Venesection is the standard first line therapy for HH to reduce end organ dysfunction [19].
Current understanding and clinical practice is variable and includes dietary restrictions, weight loss as vast majority of patients tend to have fatty liver Nonalcoholic Steatohepatitis (NASH) as a significant contributory factor. Iron overload can be associated with organ dysfunction if left untreated with potentially significant clinical manifestations [20]. Without expensive or invasive investigations, it can be difficult to identify definitively patients with true iron overload versus other causes of hyper ferritinaemia. Genetic testing for causes of non-HFE hemochromatosis is either unavailable or cost prohibitive in community based health facilities, and excluding HFE mutations does not fully exclude genetic mutations pre-disposing patients to iron overload. Hepatic MRI for iron quantification is also prohibitively expensive for most patients. Liver biopsy is often contraindicated in patients who have significant co-morbidities and is an invasive procedure with associated morbidity and mortality.
Once secondary causes of hyper ferritinaemia, as well as HFE gene mutations, are excluded, some patients may have persisting high ferritin levels with signs of iron overload, including associated end organ dysfunction such as abnormal Liver Function Tests (LFT). In this non-HFE hyper ferritinaemia patients who have unclear a etiology, prognosis and treatment decisions are made difficult for the clinician. This study aims to clarify the efficacy of available treatments in preventing iron overload, including venessection and lifestyle measures, in patients in the community, who have had HFE gene mutations and other secondary causes of hyper ferritinaemia excluded.
Patients with elevated ferritin levels of ≥ 300 ug/L, without HFE DNA abnormality (C282Y, H63D or S65C), were invited to participate in the study between 2016-2020 (n=144). Polymerase chain reaction testing was used to confirm HFE gene mutation status. Patients with known chronic liver disease, malignancy, active hepatitis, pregnancy, transfusion dependent illnesses, active infection or inflammatory disorders were excluded. Tumor markers and a CT scan of the chest, abdomen and pelvis were performed to exclude malignancies and an MRI scan was performed for patients with indeterminate findings. The final cohort of patients, who satisfied the inclusion and exclusion criteria were consented for the study (n = 120). Written and informed consent was obtained for participation in the study.
All participants underwent routine laboratory testing including Full Blood Count (FBC), Renal Function and Electrolytes (UEC), Liver Function Tests (LFT), Erythrocyte Sedimentation Rate (ESR), Thyroid Function Tests (TFT) and Plasma Glucose Blood Sugar Level (BSL). Abnormal laboratory values were confirmed on two occasions 2 weeks apart at baseline to exclude transitory derangement. A detailed dietary and lifestyle history including alcohol consumption was recorded. Patients were provided with dietary education and advised to maintain alcohol intake of less than 20 g/day during the study (congruent with Australian Guidelines) and target a BMI <30 kg/m2 for a period of twelve to 24 weeks. Patients with persistently elevated ferritin levels of greater than 300 ug/L despite lifestyle modification for 24 weeks were further treated with venesection, until ferritin improved to below 300 ug/L. After the treatment, laboratory investigations (Ferritin, LFT, TFT and BSL) were repeated. A sub-group analysis was performed on patients treated with lifestyle education only versus lifestyle education and venesection to compare the data for BMI and transferrin saturations. To examine the extent of change from pre-test to post-test results, the Wilcoxon test was used for non-normally distributed continuous variables, whereas the McNemar test was used for dichotomous variables [21-23]. A p-value of less than 0.05 was considered to be statistically significant. Data were analyzed using SAS V.9.4 software.
Statistical analysis
The Wilcoxon signed-ranks test was used to examine the extent of change on ferritin levels from pre- to post-intervention. A sub-group analysis was carried out by sex (male versus female) and type of treatment (patients treated with lifestyle education only versus lifestyle education and venesection). A p-value of less than 0.05 was considered to be statistically significant. Data were analyzed using SAS V.9.4 software [24].
Of the 144 participants who were screened for inclusion in the study, twenty four patients were excluded due to malignancy or an acute illness or other causes of hyper ferritinaemia. The final cohort consisted of 120 patients, of which 97 (80.8%) were male and 23 (19.2%) were female. The mean age was 64 years (29-88), 63 years (29-88) for males and 68 years (47-79) for females. As shown in Table 1, the majority were males (80.8%) and more than half (59.2%) underwent venesection therapy. The mean age was 64 years. For the overall sample (N=120), ferritin levels decreased significantly from pre-treatment to post-treatment as indicated by the Wilcoxon signed-ranks test (p<.001).
| Characteristic | Mean age |
|---|---|
| Male n(%) | 97 (80.8) |
| Female n (%) | 23 (19.2) |
| Baseline BMI; 25, n (%) | 66 (85.7) |
| Venesecti on therapy n (%) | 71 (59.2) |
Table 1: Ferritin levels decreased significantly from pre-treatment to post-treatment as indicated by the Wilcoxon signed-ranks test (p <.001).
Body mass index
The median pre-treatment BMI of the patients was 29 kg/m2 (19.70-44.30 kg/m2), 29.05 kg/m2 for males and 28.30 kg/m2 for females. Post therapy median BMI was 28.40 kg/m2 (21.30-44.30 kg/m2), 28.50 kg/m2 for males and 27.80 kg/m2 for females. 40.8% of patients (49 of 120 patients) demonstrated an improvement in BMI after treatment, 39.2% (47 of 120 patients) did not have a change in their BMI after treatment, and 20% (24 of 120 patients) had an increase in BMI. 46 of 120 (38.3%) patients were obese (BMI>30), 29 (63.0%) of which required venesection therapy in addition to life-style modifications and 17 (37.0%) had improvement in laboratory values with lifestyle modifications only (Table 2).
| BMI (Kg/M2) | Venesection therapy | No venesection therapy | Total |
|---|---|---|---|
| >30 | 29 | 17 | 46 |
| <30 | 42 | 32 | 74 |
Table 2: Median pre-treatment BMI of the patients.
74 of 120 (61.7%) patients were non-obese (BMI<30), 42/74 of which (56.8%) required venesection therapy in addition to lifestyle modifications and 32/74 (43.2%) had improvement in laboratory values with lifestyle modifications only.
Ferritin
The mean pre-treatment ferritin level was 880 ug/L (327-6630); mean of 875 ug/L (402-2907) for males and 885 ug/L (327-6630) for females. All patients had a reduction in their ferritin pre- (peak) and post-treatment. Post-treatment, the median ferritin was 307 ug/L (100-1384); 312 ug/L (100-1384) for males and 299 ug/L (117-1372) for females.
Table 3 presents the results of the comparison of pre- and post-treatment levels of ferritin accounting for participants’ sex and venesection. Males with (n=57) and without venesection (n=38) showed a significant reduction of ferritin from pre- to post-treatment (p-values <.001). Similarly, females who received (n=13) and did not receive venesection (n=11) also showed a significant reduction in their ferritin levels from pre- to post-treatment (p-values ≤ .001).
| Ferritin | N | Mean | Median | SD | p-value |
|---|---|---|---|---|---|
| No venesection | 38 | ||||
| Pre-treatment | 895.5 | 823.5 | 447.5 | <.001 | |
| Post-treatment | 330.4 | 286 | 205.2 | ||
| venesecti on | 58 | ||||
| Pre-treatment | 982.4 | 877 | 442.5 | <.001 | |
| Post-treatment | 394.8 | 319 | 217.9 | ||
| No venesection | 11 | ||||
| Pre-treatment | 768.5 | 616 | 395.3 | <.001 | |
| Post-treatment | 255.4 | 205 | 133.3 | ||
| venesecti on | 13 | ||||
| Pre-treatment | 1075.8 | 925 | 422.2 | <.001 | |
| Post-treatment | 383.8 | 328 | 168.1 |
Note: SD=Standard deviation awilcoxon signed-ranks test.
Table 3: Reduction in their ferritin levels from pre- to post-treatment (p-values ≤ .001).
LFTs (gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase)
Pre-treatment, 74 of 120 (61.7%) patients had abnormal LFTs, of which 60 were males (representing 61.9% of male population) and 14 were females (representing 60.9% of female population).
Pre-treatment, 46 of 120 patients (38.3%) had normal LFTs, of which 37 were males (representing 38.1% of male population) and 9 were females (representing 39.1% of female population).
Post-treatment, 22 (18.3%) had abnormal LFTs, of which 16 were males (representing 16.5% of male population) and 6 were females (representing 26.1% of female population).
Post-treatment, 98 (81.7%) had normal LFTs, of which 81 were males (representing 83.5% of male population) and 17 were females (representing 73.9% of female population).
Thyroid Function Test (TFT)
Subjects were instructed to document joint discomfort and duration of joint discomfort after their regular sporting activity in a daily diary. From this diary, the mean joint discomfort over 14 days before each study visit was calculated. No significance was observed between the study groups at baseline for the pain duration (p>0.05). A significant difference in the duration of pain after sporting activity over baseline was observed in Undenatured Collagen and PLA groups (p<0.05). When performing the subgroup analysis, a significant decrease of pain after sporting activity over baseline in subgroup of subjects aged 20-35y old in Un denatured Collagen group p<0.05, Table 5 was observed, while no such significance was seen in PLA group (p>0.05).
Blood Sugar Level (BSL)
Pre-treatment, 22 (18.3%) patients had abnormal BSL, of which 19 were males (representing 19.6% of male population) and 3 were females (representing 13.0% of female population).
Pre-treatment, 98 (81.7%) had normal BSL, of which 78 were males (representing 80.4% of male population) and 20 were females (representing 87.0% of female population).
Post-treatment, 11 (9.2%) had abnormal BSL, of which 11 were males (representing 11.3% of male population) and no females had abnormal BSL.
Post-treatment, 109 (90.8) had normal BSL, of which 86 were males (representing 88.7% of male population) and 23 were females (representing 100% of female population).
Transferrin saturation
Pre-treatment median transferrin saturation was 45%, with males 45% and females 43%. Post-treatment median transferrin saturation was 30%, with males 31% and females 27%.
Regardless of the cause, it is well established that iron overload can cause complications for patients including end-organ dysfunction, as reflected in liver, pancreatic and thyroid function testing [25]. In theory, patients with iron overload not associated with genetic mutation or blood transfusion should see improvement in laboratory markers with simple lifestyle modifications in most cases. Unfortunately, patients often either cannot maintain the long-term lifestyle changes necessary to show improvement, or despite required changes made, they continue to experience associated clinical manifestations of iron overload [26-28]. These patients are therefore still at risk of morbidity if left untreated.
In our pilot study, conservative measures of lifestyle modifications did not always result in a satisfactory outcome, with 49 patients (40.8%) showing response and 71 patients (59.2%) required additional venesection therapy to achieve improvement of end organ dysfunction. End organ dysfunction was assessed from liver, pancreatic and thyroid dysfunction point of view with biochemistry analysis. The response to intervention was most notable in men, demonstrating significant improvements in liver function with additional venesection therapy. Table 4 shows a statistically significant improvement in abnormal LFTs in males in both groups of patients on lifestyle modifications and those patients who required additional venesection therapy, but not in females. Statistically significant changes are not noted for other measures of pancreatic or thyroid dysfunction.
| Blood Sugar Level (BSL) and Thyroid Function Studies (TFT) | Pre-test | Post-test | Related- samples McNemar change test | p-value |
|---|---|---|---|---|
| MALES | ||||
| No venesection (n=37) | ||||
| Abnormal LFT | 23 (62.2) | 5 (13.5) | 14.45 | <.0001 |
| Abnormal BSL | 9 (24.3) | 5 (13.5) | 2.25 | 0.125 |
| Abnormal TFT | 1(2.7) | 0 | - | - |
| Venesection (n=60) | ||||
| Abnormal LFT | 37 (61.7) | 11(18.3) | 22.321 | <.0001 |
| Abnormal BSL | 10 (16.7) | 6 (10.0) | 2.25 | 0.125 |
| Abnormal TFT | 4 (6.7) | 0 | 2.25 | 0.125 |
| FEMALES | ||||
| No venesection (n=12) | ||||
| Abnormal LFT | 7 (58.3) | 3 (25.0) | 2.25 | 0.125 |
| Abnormal BSL | 2 (16.7) | 0 | 0.5 | 0.5 |
| Abnormal TFT | 2 (16.7) | 0 | 0.5 | 0.5 |
| Venesection (n=11) | ||||
| Abnormal LFT | 7 (63.6) | 3 (27.3) | 2.25 | 0.125 |
| Abnormal BSL | 1(9.1) | 0 | - | - |
| Abnormal TFT | 2 (18.2) | 0 | 0.5 | 0.5 |
Table 4: Statistically significant improvement in abnormal LFTs in males and females.
It is well proven in the literature that iron overload is associated with liver function abnormalities and possibly cardiac diseases and colon malignancies [29]. In some of our patients, lifestyle modifications alone showed significant improvement in ferritin readings and abnormal LFTs. In those patients who failed to improve with lifestyle modifications alone, the addition of venesection therapy precipitated significant improvement in both ferritin and LFTs.
While our study suggests there is benefit to lifestyle interventions, it should be noted that only 38% of patients with a BMI>30 responded adequately and 63% of these patients required additional venesection therapy, potentially highlighting that lifestyle measures alone are not always effective, or compliance may prove difficult. Furthermore, 58% of non-obese patients required additional venesection therapy, indicating there may be additional heritable or genetic factors contributing to hyperferritinaemia. Approximately two-thirds patients who required venesection therapy were non-obese.
Venesection therapy is inexpensive, safe, and can be performed on an outpatient basis. It should be considered in all patients who fail to improve on lifestyle and dietary modification strategies. Hence, we propose for male patients with negative HFE gene mutation testing that once alcohol excess, non-alcoholic fatty liver disease, malignancy, infection, and other inflammatory causes have screened negative, trial of lifestyle modifications including calorie controlled diet, exercise and BMI reduction, be initiated. If these measures fail for any reason after 3-6 months, trial of venesection therapy may be initiated (Figure 1).
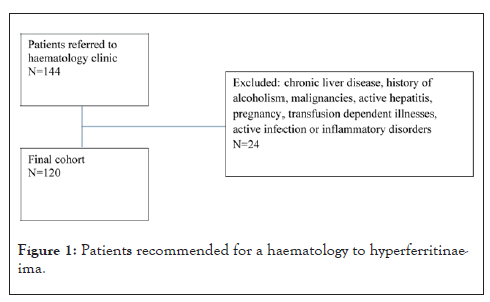
Figure 1: Patients recommended for a haematology to hyperferritinaeima.
European guidelines in managing such patients have recommended an algorithm for an approach to hyperferritinaeima [30] and we have proposed a modified algorithm based on our study findings, as outlined in Figure 2.
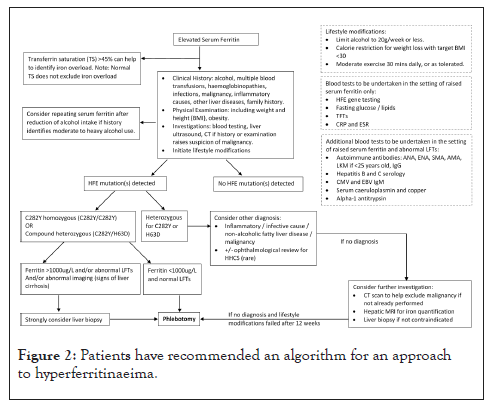
Figure 2: Patients have recommended an algorithm for an approach to hyperferritinaeima.
The major limitation in this study is its single arm: there was no control group for comparison. This was due to considered ethical implications of failure to initiate treatment for iron overload with the potential for irreversible organ damage without treatment (Figure 3). Hence historical control data was used [31].
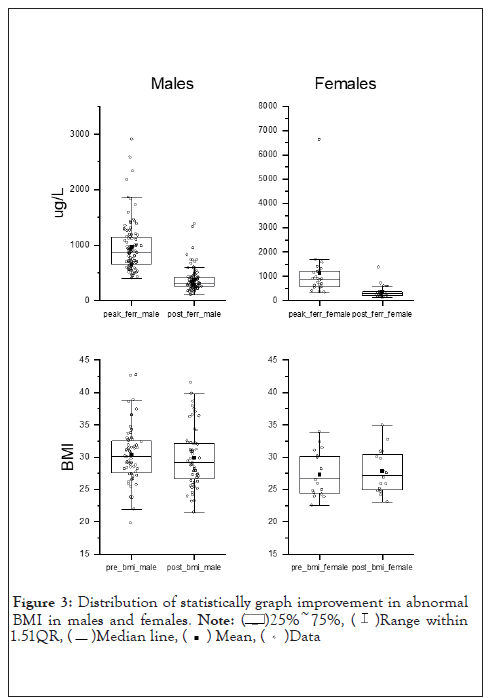
Figure 3: Distribution of statistically graph improvement in abnormal BMI in males and females
 .
.
Hyperferritinaemia in patients who do not have common HFE hemochromatosis mutations or other known causes benefit from a combination of life style modifications and additional venesection therapy, especially men. Clinical and laboratory benefits are significant with these minimally invasive interventions. The literature that iron overload is associated with liver function abnormalities and possibly cardiac diseases and colon malignancies. Hence historical control data was used. The majority of patients were men, which has skewed our findings. A larger cohort study including an equal proportion of women is planned to address this issue of applicability of results to women.
Illawarra Private Cancer Cancer Care Centre Institutional Review Board approved this study.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Middela A, Ramakrishna S, Raman A, Ramakrishna R, Alexander W, Cuenca J, et al. (2022) Clinical Outcomes and Management of Patients with Non-HFE Hyperferritinaemia: A Pilot Study. J Clin Chem Lab Med. 5:251.
Received: 31-Oct-2022, Manuscript No. JCCLM-22-19911; Editor assigned: 04-Nov-2022, Pre QC No. JCCLM-22-19911 (PQ); Reviewed: 18-Nov-2022, QC No. JCCLM-22-19911 ; Revised: 25-Nov-2022, Manuscript No. JCCLM-22-19911 (R); Published: 02-Dec-2022 , DOI: 10.35248/JCCLM.22.5.251
Copyright: © 2022 Middela A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.