Clinical Pediatrics: Open Access
Open Access
ISSN: 2572-0775
ISSN: 2572-0775
Research Article - (2022)Volume 7, Issue 6
Back ground: Systemic lupus erythematosus (SLE) is a chronic autoimmune multi-systemic inflammatory disorder that starts at childhood in 10%-20% of patients. There is paucity of reports of childhood-onset SLE (cSLE) from developing countries, with little data from Southeast Asia. The purpose of this study is to describe the clinical features at onset and disease manifestation patterns of Filipino patients with cSLE seen in a tertiary hospital in the Philippines.
Methods: This study included patients diagnosed with SLE (age of onset<18 years) seen in the Section of Rheumatology from 2008 to 2019. In-patient and out-patient clinic files of the study population were reviewed. Data collected included demographic profile as well as clinical and laboratory manifestations at initial presentation and during disease course.
Results: A total of 304 cases of cSLE were reviewed. Predominantly affected were females (89.1%). The median age at diagnosis was 13 years (range 3-18 years). The most common presenting features were fever (48.4%), anemia (45%), and arthralgia (40.1%). Predominantly affected systems at diagnosis were hematologic (56.9%), mucocutaneous (55.9%), musculoskeletal (43.4%), and renal (42.1%). Disease activity at diagnosis was generally high (average SLEDAI-2K 12.8). In terms of disease damage, the most common organ damage accrued was renal (20.7%), neuropsychiatric (8.6%), and musculoskeletal (4.6%). All patients were given corticosteroids and hydroxychloroquine, while the most common therapy used for renal disease was intravenous cyclophosphamide.
Conclusion: Among Filipino cSLE patients, the most common features at disease onset and diagnosis were fever, anemia, and arthralgia. Renal involvement occurs in 67.8% of patients throughout the disease course, and is the most predominantly affected in terms of disease damage. Clinical and laboratory features observed were relatively similar to cSLE patients from other series.
Childhood-onset; Systemic lupus erythematosus; Phenotype
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune inflammatory disorder that is multi-systemic with a complex etiology involving interplay of genetic, hormonal and environmental factors. Clinical manifestations, as well as its complications and outcomes, vary among patients. Females are predominantly affected, and although most commonly diagnosed during the second to fourth decades of life, disease onset can occur at any age. Disease starts at childhood in 10–20% of patients [1]. Although found worldwide, its prevalence varies with ethnicity and socio-economic status. The prevalence of SLE is significantly greater in African-American, Asian, Hispanic, and North American Indian children than in Caucasians [2]. Presentation of childhood-onset SLE (cSLE) also varies among different ethnic backgrounds [3-8]. Several clinical symptoms of SLE are more commonly found in children than in adults. Although it is fundamentally the same disease, it is generally accepted that children with SLE have greater disease severity and earlier accumulation of disease damage than adults with SLE. There is a paucity of reports of cSLE from developing countries. Furthermore, there is little data from Southeast Asia, specifically the Philippines, on the profile of cSLE and its outcome.
This study was conducted to provide local data that will present and summarize the clinical manifestations of SLE in Filipino children. Having an index of how the disease would present in the local setting will make clinicians more aware of the strong possibility of SLE in a child who presents with those signs and symptoms. In a more homogeneous subset of patients, like in cSLE, adequate working knowledge together with an increased awareness of the disease in all its diversities will guide clinicians in early disease recognition.
Study population and sampling
This is a descriptive single center retrospective study of all patients <18 years old with cSLE, diagnosed and seen at the Rheumatology Clinic in the University of Santo Tomas Hospital (USTH) from 2008 to 2019. All patients included in the study had to fulfill the Systemic Lupus International Collaborating Clinics (SLICC) and/or the American College of Rheumatology (ACR) classification criteria for SLE. Patients who were seen first at the clinic but had no subsequent follow-up visit were excluded from the study. Data from the patients’ paper charts and Electronic Medical Records (EMR) were retrieved and reviewed. All data reviewed were anonym zed. For each subject, a data collection form was used to collect information on demographic profile (sex, age at diagnosis and during data collection, disease duration), presenting clinical and laboratory manifestations (constitutional symptoms, mucocutaneous manifestations, musculoskeletal, renal, pulmonary, cardiac, neurologic, hematologic, gastrointestinal involvement, immunologic profile), disease damage (based on the SLICC/ACR Damage Index), and medications given during the course of disease.
Measures
Recorded clinical features and laboratory findings were used to determine the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2k) score [9]. This score is widely used to assess and measure lupus disease activity by providing numerical scores based on a count of laboratory and clinical symptoms. Disease damage on the other hand was assessed using the SLICC/ ACR damage index [10]. This damage index contains several items that represent and measure organ system damage resulting from SLE disease activity and its treatment.
Statistical analysis
The demographic data, clinical and laboratory features, disease damage, and medications were analyzed using descriptive statistics. Data were expressed as median or mean for continuous variables and frequencies/percentages for categorical variables.
Demographics and characteristics
A total of 304 patients were identified and included in the study. Table 1 shows the demographics of patients included. Predominantly affected were females, with a female to male ratio of 8.2:1 (271 girls, 33 boys). Sex ratio varied across different age clusters with a lesser female predominance in the older age bracket (13-18 years old). The overall median age at SLE diagnosis was 13.0 years, with a range of 3-18 years. The median age at diagnosis for males was slightly older at 14.0 years as compared with females at 13.0 years. The median number of months from onset of symptoms to the diagnosis of SLE was 3 months, ranging from 1 month to as long as 84 months. Delays in diagnosis, from the time of symptom onset, were mostly from patients who initially presented as having chronic Idiopathic Thrombocytopenic Purpura (ITP) (Table 1).
| Patient characteristics | Number of patients (N=304) n (%) |
|---|---|
| Sex | |
| Female | 271 (89.1) |
| Female: Male ratio | 8.2:1 |
| 0-5 years old | 9:0 |
| 6-12 years old | 10.4:1 |
| 13-18 years old | 6.9:1 |
| Median age at diagnosis (years) | 13 (3-18) |
| Duration of illness, median | - |
| Onset of symptoms to diagnosis (months) | 3 (1-84) |
| Diagnosis to data collection (years) | 7 (1-12) |
Table 1: Demographic profile of cSLE patients in USTH from 2008-2019.
Clinical and laboratory features
The presenting clinical and laboratory features at diagnosis are summarized in Table 2. The most frequent presenting features were fever (48.4%), anemia (45%), and arthralgia (40.1%). Prevalence of SLICC classification criteria for SLE were determined, with a median SLICC score of 8 (range 2-10). The most frequently observed criteria at diagnosis were immunologic/serologic features (98.4%), hematologic (56.9%), and mucocutaneous features (55.9%). General symptoms like fever, easy fatigability and weight loss were present at diagnosis in 53.6% of patients (Table 2).
| Clinical and laboratory presenting features | Number of patients (N=304) n (%) |
|---|---|
| Constitutional | |
| Fever | 147 (48.4) |
| Easy fatigability | 39 (12.8) |
| Weight loss | 28 (9.2) |
| Mucocutaneous | - |
| Malar rash | 108 (35.5) |
| Discoid rash | 19 (6.3) |
| Photosensitivity | 10 (3.3) |
| Oral ulcers | 73 (24) |
| Alopecia | 54 (17.8) |
| Subacute cutaneous lupus | 2 (0.7) |
| Panniculitis | 1 (0.3) |
| Livedo reticularis | 9 (3) |
| Vasculitis | 39 (12.8) |
| Raynaud’s phenomenon | 3 (1) |
| Hematologic | - |
| Anemia | 137 (45) |
| Direct Coomb’s test positive | 14 (4.6) |
| Leukopenia | 61 (20) |
| Lymphopenia | 11 (3.6) |
| Thrombocytopenia | 59 (19.4) |
| Renal | |
| Proteinuria | 121 (39.8) |
| Nephrotic | 22 (7.2) |
| Hematuria | 61 (20) |
| Pyuria | 23 (7.6) |
| Musculoskeletal | - |
| Arthralgia | 122 (40.1) |
| Arthritis | 83 (27.3) |
| Myositis | 1 (0.3) |
| Gastrointestinal | |
| Hepatomegaly | 8 (2.6) |
| Transaminitis | 24 (7.9) |
| Splenomegaly | 5 (1.6) |
| Serositis | - |
| Pleuritis | 10 (3.3) |
| Pericarditis | 20 (6.6) |
| Neurologic | - |
| Seizure | 2 (0.7) |
| Psychosis | 3 (1) |
| Headache | 4 (1.3) |
| Peripheral neuropathy | 1 (0.3) |
| MRI Abnormality | 1 (0.3) |
| Immunologic | |
| ANA | 272 (89.6) |
| Anti-dsDNA | 222 (73.1) |
| Anti-Ro | 33 (10.9) |
| Anti-La | 20 (6.5) |
| Anti-Smith | 36 (11.9) |
| Anti-RNP | 44 (14.4) |
| Low C3 | 65 (21.4) |
| Antiphospholipid antibodies | 7 (2.3) |
| Lupus anticoagulant+ | 5 (1.6) |
| Anticardiolipin IgG+ | 4 (1.3) |
| Anticardiolipin IgM+ | 2 (0.7) |
| Anti β2 Glycoprotein 1+ | 1 (0.3) |
| SLICC Index | - |
| Median (range) score | 5 (2-10) |
| SLEDAI-2k Score | 12 (2-31) |
| Median score for males | 17 |
| Median score for females | 16 |
Table 2: Clinical and laboratory features among the cSLE patients at diagnosis.
Mucocutaneous involvement was seen in 55.9% of patients. Malar rash (35.5%) and oral ulcers (24%) were the most common mucocutaneous features. Most prevalent systemic involvement at time of diagnosis were hematologic abnormalities, found in 56.9% of patients, with anemia as the most commonly identified (45%). Thrombocytopenia was seen in 19.4% of patients, mostly occurring around the time of diagnosis. Some of these patients were initially diagnosed as dengue fever. In seven of the patients, the thrombocytopenia predated the definite diagnosis of SLE by months and even years (mean 21 months, ranging from 5 months to 4 years).
Looking at cumulative frequencies of systemic involvement, a total of 206 patients (67.8%) had renal involvement. Predominant manifestations at time of diagnosis were proteinuria (39.8%) and hematuria (20%). Twenty-four of them (11.7%) underwent renal biopsy. Biopsies were assigned to WHO classes as follows: minimal change (class I) in 1 (4.2%); mesangial glomerulonephritis (class II) in 2 (8.3%); focal segmental proliferative glomerulonephritis (class III) in 6 (25%); and diffuse proliferative glomerulonephritis (class IV) in 13 (54.2%). Two patients (8.3%) had class III and IV on biopsy, while none showed membranous glomerulonephritis (class V).
Cumulatively, neuropsychiatric manifestations occurred in 6.6% of patients, with lupus headache as the most common. Other manifestations included seizures, psychosis and peripheral neuropathy. Three patients had MRI abnormalities which included white matter hyperintensities in a patient with communicating hydrocephalus, posterior reversible encephalopathy in a hypertensive patient with seizures, and right middle cerebral artery irregularities in a patient with optic neuritis.
Serositis was seen in 8.6% of patients, with pericarditis seen more frequently. Respiratory and gastrointestinal involvement was not so common in our cohort of patients. One patient presented with pulmonary fibrosis. The more common gastrointestinal manifestations were transaminitis and hepatomegaly.
Disease activity was evaluated by the computation of the SLEDAI- 2K score. Its average at the time of diagnosis was 12.8 (range 2-31), indicating moderate to high disease activity. Males had slightly higher mean SLEDAI scores at diagnosis compared to females.
Disease damage
Patients with recorded damage were identified using the SLICC/ ACR Damage Index. The predominant organ affected in the damage index was the kidney (20.7%), mainly presenting with nephrotic- range proteinuria (Figure 1). This was followed by neuropsychiatric damage (8.6%) with manifestations including seizures, psychosis, and peripheral neuropathy. The third highest damage category was in the musculoskeletal system, mostly all due to avascular necrosis (13/14). In total, only 1.0% of patients had secondary amenorrhea. There were no reported cases of secondary malignancies.
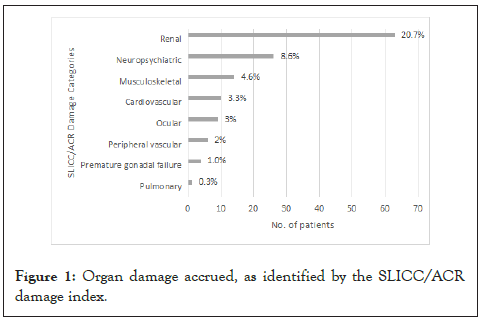
Figure 1: Organ damage accrued, as identified by the SLICC/ACR damage index.
Medications
The medications given to our patients throughout the disease course are summarized in Figure 2. All patients were given corticosteroids (oral prednisone, oral methylprednisolone, intravenous methylprednisolone) and hydroxychloroquine. Most common therapy used for renal disease was intravenous Cyclophosphamide (IV CYC), followed by Mycophenolate Mofetil (MMF). IV CYC was also used for patients with CNS disease. Rituximab (RTX) was used in 19 patients, mostly renal disease (15/19), the rest for refractory thrombocytopenia. Methotrexate was given to patients with persistent arthritis. Infliximab and etanercept were given to a patient with psoriasis. IVIG was given to one patient with refractory thrombocytopenia (Figure 2).
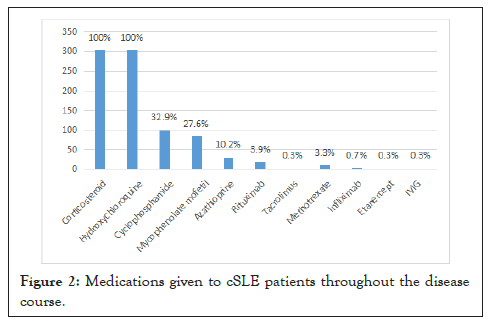
Figure 2: Medications given to cSLE patients throughout the disease course.
In our cohort of patients, demographic data were comparable with other published literature describing cSLE cohorts in Asia [11-13]. Our large cohort exhibited similar female to male ratios, along with the typical female preponderance owing to the role of hormonal factors in lupus disease expression. Females represented 89.1% of the cases with a female to male ratio of 8.2:1, which is well within the common incidence reported for cSLE in asian populations [11-13]. The female to male ratio in cSLE is generally found to be significantly less than in aSLE. A higher proportion of female patients is often reported in aSLE, with 96% female patients (female to male ratio of 23.9:1) reported in an adult Filipino SLE population [14]. Age-related gender differences normally seen in other pediatric cohorts were similarly seen in our cohort, with increasing ratios observed in those with pubertal or post-pubertal onset [12,15,16].
Comparable to other studies as well is the mean age at diagnosis at 13 years old. We reported 3 years old as the youngest age at initial diagnosis. This again is similar to other series but relatively younger compared to a previously published study in the Philippines which reported the youngest age at 8 years old [11].
The frequencies of systemic involvement at diagnosis are summarized in Table 3. In our patients, the most common manifestations at the time of diagnosis were fever, anemia, and arthralgia. Fever as a common presenting manifestation is comparable to data described by local, as well as Malaysian and Portuguese cohorts [11,12,17]. However, contrary to the Filipino cSLE cohorts described in previously reported studies, malar rash and photosensitivity were not as common in our patients [11,18] (Table 3).
| Patients | Present study | Gulay and Dans, 2011 | Cabral, 2013 | Aggarwal, 2018 | Lim, 2020 |
|---|---|---|---|---|---|
| No. of Patients | 304 (%) | 78 (%) | 56 (%) | 273 (%) | 141 (%) |
| Country | Philippines | Philippines | Portugal | India | Malaysia |
| Mucocutaneous | 55.9 | 91.0 | - | - | - |
| Malar rash | 35.5 | 65.3 | 10.7 | 23.5 | 56.0 |
| Discoid rash | 6.3 | 32.0 | 1.8 | - | 15.6 |
| Photosensitivity | 3.3 | 55.1 | 1.8 | 42.8 | - |
| Oral ulcers | 24.0 | 53.8 | - | 23.0 | 48.9 |
| Alopecia | 17.8 | 39.7 | - | 60.8 | 41.8 |
| Hematologic | 56.9 | 51.2 | 14.3 | - | - |
| Anemia | 45.0 | 8.9 | 1.8 | 36.1 | 22.0 |
| Leukopenia | 20.0 | 15.3 | - | 24.1 | 51.1 |
| Thrombocytopenia | 19.4 | 14.1 | 12.5 | 21.2 | 41.8 |
| Musculoskeletal | 43.4 | 53.8 | - | - | - |
| Arthritis | 27.3 | 21.7 | 41.1 | 69.2 | - |
| Renal | 42.1 | 62.8 | 1.8 | 48.7 | 39.7 |
| Neuropsychiatric | 3.6 | 30.7 | 1.8 | 22.7 | 16.3 |
| Serositis | 8.6 | 26.9 | 1.8 | 4.2 | 18.4 |
Table 3: Comparison of systemic involvement at diagnosis with other cSLE cohorts.
ANA are present in more than 99% of children with SLE19 and so in its absence, the diagnosis of SLE becomes questionable. However, as in most series, our cohort also includes a patient with negative ANA. This was a 17-year-old female who presented with alopecia, edema, and low C3. The diagnosis of SLE was confirmed with a positive anti-dsDNA. Some of the patients in this cohort no longer had ANA tests done. These are patients who initially presented with clinical manifestations that made the probability of SLE very high, hence were directly requested anti-dsDNA to confirm the diagnosis.
In general, the prevalence of antiphospholipid antibodies in SLE is about 20-60% [20]. Other cSLE cohorts studied by Lim et al. and Cabral et al. report of lower incidences at 14.9% and 16.4% respectively [12-20]. In our cohort, its incidence is much lower at 2.3%, but this is primarily because antiphospholipid antibodies are not routinely checked unless the patients present with symptoms.
Disease activity as measured with SLEDAI-2k scores was generally high at diagnosis in our cohort of patients. This corresponds to data showing that cSLE is more aggressive and severe compared to aSLE [21]. The high proportion of patients in our cohort presenting with significant major organ involvement at diagnosis is likewise seen in other Asian studies, though worse than reported in Caucasian cohorts [22,23].
One of the most important predictors of poor outcomes in lupus is renal involvement. Manifestations can vary from asymptomatic proteinuria to end-stage renal disease (ESRD). Generally consistent with other reported literature, the predominant kidney biopsy result of lupus nephritis patients in this cohort were class III and IV. Although most commonly reported, this may not be representative of the renal disease in our cohort, considering that not all patients with renal involvement have kidney biopsies done, usually due to financial constraints. For lupus nephritis, particularly class III and IV, aggressive treatment is recommended to prevent progression of disease. Overall, only 1% ended up with ESRD. This low incidence may be reflective of the prompt and adequate management of active renal disease in our center.
Several studies have looked at the pattern of accumulated damage amongst their cohorts. Across most published studies, the most frequently involved organ systems are the ocular, neurological, renal and musculoskeletal systems. Similar to the study by Tucker, et al. Similar to the study by Tucker et al. [24], Our cohort had the highest percentage of damage in the renal domain (20.7%), followed by the neuropsychiatric (8.6%) and the musculoskeletal (4.6%) systems. This is in contrast to other series where rates of renal damage were lower [25]. Based on the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index, renal organ damage is defined as either proteinuria >3 g/24 hrs, estimated GFR<50%, or end-stage renal disease. In our cohort, among the 67.8% of patients with renal involvement, 25.7% developed nephrotic-range proteinuria (>3 g/24 hrs). Only 3.4% had an eGFR of<50%, and only 1.5% had end-stage renal disease. This cohort of patients must be followed-up into adulthood considering the significance of renal damage amongst adult Asian lupus patients [25,26].
In contrast to other series, our cohort had a markedly lower rate of ocular damage (3.0%) despite having patients exposed to moderately high doses and prolonged duration of corticosteroids due to high disease activity, and universal use of hydroxychloroquine.
Therapy given to children in our study is similar to that carried out in patients from aSLE cohorts. This is reflective of how pediatric SLE management is largely based on the clinical experience and large randomized controlled trials in adults. In our cohort, more patients received IV CYC than MMF as induction therapy. The choice of induction treatment was based upon the individual physician’s choice. Previous cSLE studies show IV CYC and MMF to be comparably efficacious with regards to treatment response, damage accrual and time to next flare [27]. A prospective comparison of IV CYC versus MMF induction treatment is highly needed to better formulate future lupus nephritis treatment protocols for children.
In aSLE patients with severe or refractory disease, RTX has been used as an adjunctive therapy with good results. However, few studies have been conducted in children and none of these were randomized controlled trials [28]. RTX was given to 19 patients in our cohort, mostly for lupus nephritis but also for refractory thrombocytopenia. RTX has been shown to be safe and effective for treating the renal and hematologic manifestations of cSLE, especially in terms of disease activity, immunologic measures, and steroid-sparing effect. Unfortunately, there was failure to achieve remission in most of our lupus nephritis patients who were given RTX. This could be due to the fact that all of them started off with really bad nephritis, and all failed with CYC. Treatment failure of RTX in lupus nephritis is reported in up to 43% of patients in some studies [28]. In addition, even if remission is attained with immunosuppressive drugs, nephritis relapse can occur in 35% of responders and seems to be a determining factor in end-stage kidney disease in children [29].
Several medications used in the treatment of cSLE may produce severe complications including corticosteroid-related adverse effects and infections from cyclophosphamide. Considering how the medications given may play a factor in the development of infections in SLE, the balance between benefits and side effects should always be considered in selecting options for cSLE treatment. For both children and adults with SLE, infection remains the most common cause of morbidity and mortality [30], as was observed in our patients. Severe infection, along with active disease, was the causes of mortality in our cohort. Most of these patients belonged to low income families, where poor follow-up and treatment compliance are major contributing factors.
Study limitations include its retrospective nature. Data collected and analyzed were purely sourced from information gathered in the chart review, and is highly reliant on the accuracy of documentation in patients’ records. Although our cohort is one of the largest ever studied for cSLE patients in the country and in Southeast Asia, as a single-centre study, ethnic composition of the cohort may be homogenous and therefore general applicability of data may be limited. Primarily due to financial constraints, another limitation of the study was the inability to have complete antibody profiles tested for all patients. The same way kidney biopsies were not done in all patients with renal involvement. This may account for variations in the reported frequencies among different studies.
Among Filipino cSLE patients, the most common features at disease onset and diagnosis were fever, anemia, and arthralgia. Renal involvement occurs in 67.8% of patients throughout the disease course, and is the most predominantly affected in terms of disease damage. Clinical and laboratory features observed were relatively similar to cSLE patients from other series.
Ethics approval and consent to participate
The research was conducted upon the approval of the University of Santo Tomas Research Ethics Committee in accordance with the ethical principles set out in the Declaration of Helsinki 2015 WHO guidelines, International Conference on Harmonization – Good Clinical Practice. This study likewise complied with principles stated in the National Ethics Guidelines for Health and Health-Related Research (NEGHHRR) 2017 Edition, specifically the Ethical Guidelines For Research Involving Minors or Children.
Consent for publication
The principal investigator respectfully requested the University of Santo Tomas - Research Ethics Committee to waive the consent form in this study. This is in accordance to the NEGHHRR 2017 Edition which states that informed consent may be waived when the study involves archival research involving publicly available documents.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors have nothing to disclose. No conflicts of interest or biases were identified.
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Borromeo JA, Collante MTM, Bernal CB (2022) Clinical Phenotype of Filipino Childhood-Onset Systemic Lupus Erythematosus:12-Year Study in a Tertiary Hospital. Clin Pediatr. 7:224.
Received: 01-Nov-2022, Manuscript No. CPOA-22-16965; Editor assigned: 03-Nov-2022, Pre QC No. CPOA-22-16965 (PQ); Reviewed: 17-Nov-2022, QC No. CPOA-22-16965; Revised: 23-Nov-2022, Manuscript No. CPOA-22-16965 (R); Published: 30-Nov-2022 , DOI: 10.35248/2572-0775.22.7.224
Copyright: © 2022 Borromeo JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.