Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Mini Review - (2024)Volume 15, Issue 5
The EuroGuiDerm guideline on Lichen Sclerosus (LS) was developed by 34 interdisciplinary experts from 16 countries, following the EuroGuiDerm Methods Manual v1.3 and was published in 2023. The guideline provides evidence-based and consensus-based guidance on diagnostics, treatment, patient education, follow-up, and interdisciplinary management among other aspects. This article briefly summarizes the most important recommendations of the guideline. In addition, particular attention is paid to the difficulties that arise in the care of LS patients in clinical practice. LS is a chronic inflammatory skin disease that primarily affects the anogenital site in both sexes and can occur at any age. Nonspecific symptoms, such as itching and pain, along with initial nonspecific clinical changes, often lead to a delayed diagnosis. As a result, patients often remain undiagnosed and untreated for a long time, which leads to irreversible damage such as scarring, atrophy and possibly cancer development.
Lichen sclerosus; Clinical guidelines; Patient care
The guideline recommends potent or very potent topical corticosteroids as first line therapy, without specifying active ingredients. Initially, topical corticosteroids should be applied once a day. Maintenance therapy at a lower frequency is advised once symptoms and clinical signs are controlled. Circumcision in case of male patients with LS related phimosis or topical calcineurin inhibitors are secondary options. Laser therapy, including CO2, ER: YAG and Nd: YAG lasers, and injections with platelet rich plasma are also discussed in the guideline but not recommended due to inconsistent regimens and low quality evidence. While the EuroGuiDerm guideline offers comprehensive recommendations on the management of LS patients, treating this anogenital dermatosis remains challenging. More high-quality research on effective treatments and LS pathomechanisms is needed.
In 2023, the updated EuroGuiDerm Guideline on Lichen Sclerosus (LS) [1,2], was published. 34 experts (dermatologists, gynecologists, urologists, sexologists, pediatric surgeons, histopathologists and patient representatives) from 16 countries were involved. The guideline was developed in accordance with the EuroGuiDerm Methods Manual v1.3, which corresponds to an S3 level. The guideline consists of an evidence-based part, which includes the treatment chapters where the evidence data was systematically analyzed, and a consensus-based part, which addresses symptoms and accompanying complaints, diagnostics, patient education, follow-up, and interdisciplinary management, among other aspects.
In the following, we summarize the most important parts of the guideline, focusing on the difficulties identified in the care of patients with LS. LS is a chronic inflammatory skin disease that usually affects the anogenital site (Figure 1). LS can affect both males and females and can occur at any age. The chronic inflammation initially leads to nonspecific skin changes like redness, edema, fissures, erosions or hyperkeratosis, which in female patients often affect the labia minora, the interlabial sulcus, the perineal or perianal area and in male patients the glans penis, the coronary sulcus, the frenulum and the foreskin, less frequently the meatus urethrae and the urethra. Advanced LS will results in sclerosis leading to tight, non-elastic tissue and atrophy or resorption of the affected structures. In females, shortening of the labia minora or adhesions may occur, leading to narrowing of the vaginal introitus or a "buried clitoris". In male patients, secondary phimosis, narrowing of the meatus and strictures of the urethra may complicate LS.
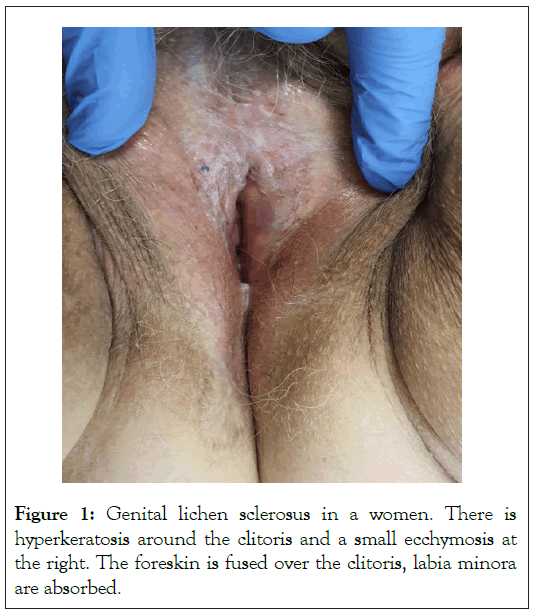
Figure 1: Genital lichen sclerosus in a women. There is hyperkeratosis around the clitoris and a small ecchymosis at the right. The foreskin is fused over the clitoris, labia minora are absorbed.
Genital itch (especially in female patients) or pains (especially in male patients) are the predominant symptoms. Accompanied dyspareunia and dysuria reduce the quality of life severely. Perianal involvement, which is often seen in girls, can lead to pain-related obstipation. However, LS can also be asymptomatic. Very little is known about bladder, bowel, and pain symptoms that may precede the skin changes of LS or develop over the course of the disease. They do not seem directly related to the structural changes of LS and do not improve with adequate topical anti-inflammatory therapy. In women, this pain may be classified as secondary vulvodynia. The pathomechanism is not known and standardized treatments for these symptoms don’t exist. Pain treatment using topical or systemic analgesics, like topical lidocaine and low dose tricyclic antidepressants (amitriptyline and trimipramine) are helpful. Laser treatment used for example in postmenopausal vaginal atrophy is unlikely to improve those symptoms.
The diagnosis of LS is usually made clinically. However, exact diagnostic criteria are not established and a major problem arises from partly non-specific signs and symptoms of LS, especially in early disease. This often results in a delayed diagnosis of LS3 and development of irreversible damage, such as scarring or atrophy of structures. This is particularly frustrating, as appropriate treatment in early disease will prevent such irreversible damage. It is therefore important that an experienced physician will see the patient in time to recognize early symptoms and clinical signs of LS. Furthermore, next to the lack of diagnostic criteria there is also no widely accepted scoring system available that will help to judge disease progression. A number of scoring systems are suggested [3-10], however, these are not agreed upon and have not found their way into clinical practice. We first need to establish diagnostic criteria that will forecast the likelihood of a diagnosis of LS according to a set of signs and symptoms, and then develop a scoring system that will enable to judge disease progression. Histological examination of the tissue can be helpful in making a diagnosis. However, it is important to biopsy the right area, if available, a whitish lesion or the end of a fissure, in an untreated (3-4 weeks treatment interruption) patient should be biopsied using a 3 mm punch biopsy after local anesthesia. A pitfall are non-specific histological changes especially in early phases of LS. Various physicians, amongst them general practitioners, dermatologists, gynecologists, urologists, or pediatricians, see LS patients. However, only a small number of those are familiar with LS and may therefore miss the diagnosis. LS needs to be considered if chronic itch or pain is reported by patients leading to a prompt specialist referral.
The standard of LS treatment are anti-inflammatory topical corticosteroids. Best data is available for topical clobetasol propionate 0.05%. However, a randomized controlled study has shown that mometasone furoate 0.01% ointment does not perform worse in direct comparison [11], the guideline recommends first-line therapy with very potent or potent topical corticosteroids without specifying the active substances; an ointment is preferred than a cream. The exact treatment regimen, including the frequency of topical corticosteroid application, must be customized/adjusted to the specific clinical situation. This can be particularly challenging for physicians with little experience, as they may seek for an exact/uniform treatment plan. However, there is neither sufficient data nor consensus within the guideline group on this matter. As a rule, the topical corticosteroid should be applied once a day for at least one month, before the frequency can be gradually reduced. However, once-daily treatment is often necessary for up to three months. The aim of the corticosteroid treatment is to achieve complete resolution of the symptoms and reversible clinical signs (edema, erythema, hyperkeratosis, fissures, and erosions) of the disease. Once this is achieved, maintenance treatment should be carried out at a lower frequency for many years. Offlabel anti-inflammatory therapy with topical calcineurin inhibitors can be used as a second choice or additional agent if topical corticosteroids are contraindicated or show little effect, however, in those cases the diagnosis has to be questioned.
If secondary phimosis occurs, circumcision with complete removal of the foreskin is recommended in males. The guideline also comments on laser therapy, which is proposed increasingly as a treatment concept for LS. Different laser systems such as CO2, ER: YAG, Nd: YAG lasers or combinations have been tested in small studies, predominantly in female patients. A randomized double-blind study showed no significant advantages of a CO2 laser over a sham laser [12]. A further study, published after the development of guideline, confirmed these data, no significant differences were found between the CO2 laser group and a low-dose laser group, which represented the placebo group [13]. Three further randomized studies compared CO2 lasers with clobetasol propionate 0.05% ointment therapy. In some cases, the CO2 laser was superior in individual outcomes [14-16]. However, all three studies showed limitations due to small test groups and the lack of blinding. Therefore, whether CO2 laser therapy is an adequate therapeutic concept in LS or whether the positive outcomes are due to a placebo effect must be evaluated in larger studies. Less data is available for ER: YAG and Nd: YAG lasers [17,18]. Overall, the guideline group came to the conclusion that, based on the data available, no recommendation can be made either for or against laser therapy at present.
Also Platelet Rich Plasma (PRP) application is gaining increasing attention in the treatment of LS. PRP is a blood product with a high concentration of platelets derived from the patient's own blood. PRP is believed to have a positive influence on healing and regenerative processes, mediated by growth factors. Good treatment outcomes in LS are reported, also in patients who are refractory to topical corticosteroids. However, most studies are small, uncontrolled, and of poor quality [19-24]. A randomized double-blind study with 30 patients suffering from vulval LS, showed no differences between the placebo group and the PRP group [25]. Based on the available data, the guideline group came to the conclusion that no recommendation for or against PRP therapy can be made at present. There is no standardized outcome assessment for LS. This makes the few studies on LS hardly comparable. Fortunately, a core outcome set is currently being developed, which shall significantly improve the study quality and comparability of the forthcoming data [26].
Overall, the new EuroGuiDerm guideline provides comprehensive guidance for many aspects relevant to the treatment of LS patients. It offers specific recommendations for anti-inflammatory therapy as well as practical hints to improve the quality of daily life of LS patients. Despite this, treating LS often remains challenging. More high-quality studies on effective management are urgently needed.
This study was funded by a grant (23214MFDS262) from the Ministry of Food and Drug Safety (MFDS) and by the KoCVAM project for ‘Establishment of national foundation for development of alternative test methods’ from 2023 to 2024.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kirtschig G, Kinberger M (2024) Clinical Practice Challenges in Lichen A Review on 2023 EuroGuiDerm Guidelines. J Clin Exp Dermatol Res. 15:675.
Received: 28-Aug-2024, Manuscript No. JCEDR-24-33032; Editor assigned: 30-Aug-2024, Pre QC No. JCEDR-24-33032 (PQ); Reviewed: 13-Sep-2024, QC No. JCEDR-24-33032; Revised: 20-Sep-2024, Manuscript No. JCEDR-24-33032 (R); Published: 27-Sep-2024 , DOI: 10.35841/2155-9554.24.15.675
Copyright: © 2024 Kirtschig G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.