
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Case Report - (2023)Volume 13, Issue 4
Accidental exposures or suicidal intent have long been the cause of heavy metal toxicity. There are very few publications that define the manifestations and effects of a variety of heavy metals. This case report seeks to highlight the case of a 27-year-old woman who presented with leukopenia, thrombocytopenia, and nephrotic range proteinuria after taking traditional medicine for a common ailment. Mercury and cadmium were detected in her urine sample, indicating that she was exposed to both heavy metals. The complete resolution of symptoms following cessation of traditional Siddha medication suggests a temporal relationship between the development of hematological and renal manifestations and exposure to heavy metals.
Mercury poisoning; Cadmium poisoning; Heavy metal poisoning; Side effects
Mercury, lead, and cadmium are the three most toxic heavy metals with clinical implications in daily practice. Despite the various measures taken to prevent accidental exposures, these heavy metals continue to be ubiquitous and pervasive environmental toxins. Mercury and other heavy metals are used as binding agents in the preparations of traditional indigenous medicine such as Siddha, Ayurveda, and integrative medicine [1]. Mercury has been shown to significantly affect the hematological milieu via direct toxicity and inflammation, as well as immunological phenomena, and its effects on the kidneys and nervous system have been well documented in animal studies [2]. Cadmium, like mercury, is a highly persistent environmental contaminant, and studies have shown that exposure to high levels of cadmium has systemic effects, including neurological, reproductive, immunological, and lymphoreticular effects [3]. It becomes vastly important in cases such as this patient where inadvertent exposure to high levels of toxic heavy metals especially in unsanctioned preparations of traditional medicine results in irreversible damage to the immune system, hematological milieu, and renal system. Our patient was fortunate to circumvent the lasting effects of these heavy metals; the clinical picture and background of this patient may help clinicians evaluate similar cases and decide how best to treat them.
27-year-old lady with a history of Allergic Bronchitis on traditional medicine (Siddha) for the past 3 months, presented with complaints of fever of 3 days duration. She had associated cough with expectoration. She also had associated 5-6 episodes of watery loose stools and vomiting lasting for 2 days prior to admission. She was admitted with a provisional diagnosis of tropical fever.
Investigations
On evaluation, she was found to have severe leucopenia of 980/ microliter with 760 neutrophils and thrombocytopenia of 95,000/microliter. Peripheral smear showed no atypical lymphocytes or blast cells, however immature cells in the form of myelocytes and metamyelocytes were noted. Her ESR and CRP were 4 and 35 respectively. Tropical fever workup with dengue serology, scrub IgM, malarial antigen test, and widal test were negative. There was no transaminitis in the liver function tests and the Chest X-ray showed no abnormalities. Coagulation parameters were within the normal range.
Renal function tests were normal. However, her D-Dimer was elevated (31.56-reference range<0.5 mg/l), LDH was 959 (reference range-<246 IU/L) and serum ferritin was>2000 ng/ml indicating accelerated cell destruction. Serial monitoring of blood cell count showed persistent and steady fall with a nadir of the absolute neutrophil count of 60/microliter and thrombocytopenia of 40,000/microliter. Considering neutropenic sepsis, she was initiated on Ceftazidime according to standard protocols.
ECHO showed normal valves and chambers, with no regional wall motion abnormalities, no vegetation, no effusion or clots, and normal LV systolic function. Viral markers such as HIV, Hepatitis B, and Hepatitis C panel were found to be negative as well as Paul Bunnel Test for Ebstein Barr Virus was also negative. COVID-RT PCR was also found to be negative. Ultrasound abdomen showed hepatosplenomegaly or any other evidence of infective foci. Her urine routine showed evidence of nephrotic range proteinuria with a urine PCR of 5.43 (normal -0.1-0.3). Blood and Urine cultures were sterile after 5 days of incubation. The stool analysis was clean.
Since she had persistent severe bicytopenia (Leukopenia and thrombocytopenia) and evidence of proteinuria, an autoimmune process was suspected. ANA-Immunofluorescence and ANA ELISA were found to be negative. However, C3 levels were low (72, reference range>80) and normal C4 levels. C-ANCA and PANCA were found to be negative.
Vitamin B12 levels and Folic Acid levels were found to be normal. Bone Marrow biopsy was done which showed hypocellular marrow with increased megakaryocytic and histiocytic activity with histiocytes ingesting nuclear debris were noted. There was no lymphocytic infiltration nor there was any sign of malignancy or infection. The possibility of heavy metal toxicity was considered in view of bone marrow suppression and nephrotic range proteinuria with a background history of ingestion of traditional medicine and a Urine Toxicology screen was sent (Figure 1).
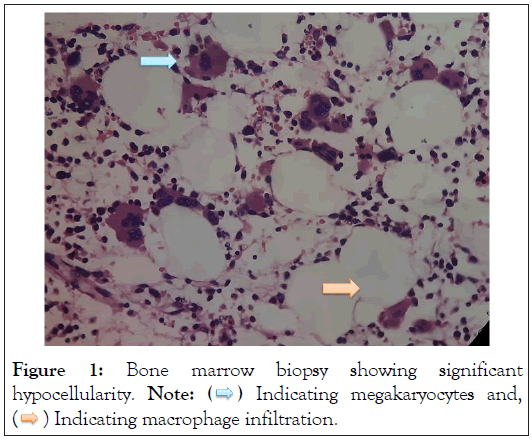
Figure 1: Bone marrow biopsy showing significant
hypocellularity. Note:  Indicating megakaryocytes and,
Indicating megakaryocytes and,  Indicating macrophage infiltration.
Indicating macrophage infiltration.
Urine Toxicology screen showed raised levels of cadmium (6.78 mcg/l, normal level-<2.60 mcg/l) and mercury of 126.04 mcg/l with normal levels less than 20 mcg/l. Hence Dual Heavy Metal Toxicity causing direct bone marrow suppression with immune mediated glomerular disease was arrived at considering the temporal association of symptoms to exposure to toxic heavy metals and resolution of symptoms with withdrawal of offending agents and adequate hydration during the hospital stay. Serial monitoring of blood counts showed leucocyte count and platelet count in an increasing trend and at follow up after 2 weeks, she had normal leukocyte and platelet counts with complete resolution of proteinuria.
There has been a steady increase in the detection of heavy metal poisonings in humans as a result of improved diagnostic techniques and clinical suspicion. Food and medication consumption, suicide attempts, industrial processes, and gold mining, to name a few, are among the numerous exposure routes. Exposure to these heavy metals as a result of increased industrial and anthropogenic activities and modern industrialization, and, to a lesser extent, their use in traditional medicine such as Ayurveda and Siddha, have caused adverse effects on human health [1]. According to the Minamata Convention, an international treaty signed in 2013 by more than 140 countries, including India, many of them are to be prohibited by 2020 [4]. The body's homeostatic physiology regulates tissue concentrations of essential elements with varying degrees of efficacy. However, the body lacks an effective homeostatic control mechanism or adaptive mechanism to prevent the potential toxicity of cadmium, mercury, and lead.
Mercury exposure causes numerous pathologies affecting multiple organ systems, including the immune system. Nevertheless, a deficiency of epidemiological studies and diagnostic criteria has hampered the consolidation of sufficient evidence to support mercury's causative role in autoimmune disease. It has been demonstrated that mercurial preparations and metal ions can stimulate inflammatory responses even in the absence of mitogens [2]. The pharmacokinetics of mercury appears to be multiphase, with a first phase lasting approximately 5 days, followed by a second phase lasting approximately 1 month, and a third phase lasting approximately 3 months [5]. In Scandinavian studies, the half-life of at least 80% of an oral dose of mercury was determined to be 42 days [6]. Mercury has also been detected in a variety of serological fluids, such as sweat, tears, breast milk, saliva, and bile, but it is primarily excreted via urine and feces [7,8].
Mercury distribution and toxicity appear to be highly differentiated, despite the substantial interindividual variability. In acute toxicity, in addition to accumulation in the GI tract, the liver, thyroid, and kidneys appear to be the most affected organs. Primarily affected in the kidneys are the glomeruli and proximal convoluted tubules [7]. Urinary mercury concentrations are typically less than 20 micrograms per milliliter, whereas our patient's concentration was 126.04 micrograms per milliliter, which is substantially higher than the toxic levels, and was also measured on the fifth day of hospitalization.
Autoantibodies have been associated with mercury exposure in a number of distinct populations [8]. The NHANES cohort studies conducted in 2007 and 2008 suggest a correlation between mercury exposure and the induction of autoantibodies, especially in cohorts with elevated mercury exposure levels [9]. Seventy-two percent of patients with renal biopsies from 26 cases were found to have glomerular disease, with membranous glomerulonephritis and minimal change disease being the most prominent pathological manifestations [10]. The mechanisms leading to mercury induced glomerular injury in humans are unknown. Mercury does exhibit substantial renal tubular toxicity, and it has been hypothesized that this results in the release of self-antigens and an inflammatory response involving the production of cytokines and autoantibodies [11].
Even though there was no evidence of autoantibody production in our patient, there was evidence of an autoimmune process as demonstrated by complement consumption and renal toxicity in the form of nephrotic range proteinuria. Although this necessitates the use of DMSA or other chelating agents, our patient's renal manifestations resolved after the withdrawal of the offending agent and adequate hydration [6].
A systematic review of 1,219 studies by Vianna, et al. [12], revealed a causal relationship between mercury exposure and hematological outcomes. Due to mercury toxicity, anemia, and less frequently leukopenia, thrombocytopenia, and pancytopenia have been reported. Anemia, lymphopenia, lymphocytosis, neutrophilia, and basophilia were reported among the 1,914 reported cases with hematological manifestations [12]. However, severe neutropenia to such a degree of 60 per microliter has never been reported. The prevalent explanation for these effects is believed to be direct toxicity to the bone marrow, as evidenced by histiocytic infiltration ingesting cell debris in the bone marrow specimen.
Regarding Cadmium, it has been demonstrated to inhibit mitochondrial oxidative phosphorylation and interfere with zinc in enzyme systems. Cadmium toxicity in humans has been described in Japan's Itai-Itai disease, which is caused by contamination of food and water sources [13]. The patients had excruciating degenerative bone disease, renal dysfunction, gastrointestinal disease, and lung disease.
Cadmium is known to result in polyuria, glucosuria, and proteinuria due to glomerulopathy and proximal tubulopathy. With chronic cadmium exposure, approximately 50% of the accumulated dose is deposited in the kidneys, and proteinuria is the first sign [14]. Intriguingly, chronic cadmium toxicity is associated with a loss of the sense of smell, which we were unable to objectively demonstrate in our patient [15,16]. The pharmacokinetics of cadmium are also multiphasic, with the fast component having a half-life of 75 to 128 days and the slow component having a half-life of 7 to 16 years [17]. Normal urinary cadmium levels are less than 2.60 mcg/l, whereas our patient had toxic levels of 6.78 mcg/l.
While it is documented that cigarette smokers have higher levels of cadmium in their bodies or as measured in their urine, levels greater than three times the normal upper limit indicate a toxic dose. Although hematological manifestations of cadmium are uncommon, its renal complications are well documented. In our patient, both Mercury and Cadmium would have contributed to the glomerulopathy. Cadmium causes renal dysfunction through its binding to Metallothionein (MT) [3,17]. This Cadmium- Metallothionein (Cd-MT) complex has a prolonged half-life, and its deposition in proximal tubular cells results in immunemediated glomerular damage.
Clinical history remains the cornerstone of diagnostic inference. High degree of suspicion should remain for patients with proteinuria and hematological manifestations. Adequate hydration and diuresis alone can resolve the manifestations in mild-moderate cases. Acute toxicity is treated with EDTA to enhance its urinary excretion. However, our patient's symptoms spontaneously resolved with adequate hydration and symptomatic treatment.
The authors declare no conflicts of interest.
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Michael RB, Mothilalraj R, Vimal J, G�?�¢??Boy R (2023) Clinical Spectrum of a Combination of Mercury and Cadmium Poisoning. J Clin Toxicol. 13:538.
Received: 19-May-2023, Manuscript No. JCT-23-24309; Editor assigned: 22-May-2023, Pre QC No. JCT-23-24309 (PQ); Reviewed: 05-Jun-2023, QC No. JCT-23-24309; Revised: 12-Jun-2023, Manuscript No. JCT-23-24309 (R); Published: 19-Jun-2023 , DOI: 10.35248/2161-0495.23.13.538
Copyright: © 2023 Michael RB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.