
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2022)Volume 13, Issue 5
Background: Although several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 (COVID-19), none have yet been shown to be efficacious.
Methods: We conducted a double-blind, randomized, placebo-controlled trial of isothymol in adults hospitalized with COVID-19 with evidence of lower respiratory tract involvement. Patients were randomly assigned to receive either isothymol (6 mg/ml) or placebo for up to 15 days. The primary outcome was the time to recovery, defined by either discharge from the hospital or hospitalization for infection-control purposes only.
Results: A double-blind, randomized, controlled trial with placebo and modified isothymol was conducted in adults who were hospitalized with COVID-19. Patients were randomized to receive isothymol (6 mg/ml) or placebo for up to 15 days. The primary outcome was recovery time, defined by discharge from the hospital. The results of the 600 patients (300 assigned to isothymol and 300 to placebo) with data available after randomization suggest that those who received isothymol had a median recovery time of 7 days (95% confidence interval [CI], 5 to 9), compared with 14 days (95% CI, 11 to 15) in those who received placebo (recovery rate ratio, 1.24; 95% CI, 0.78 to 1.87; P<0.001). The Kaplan-Meier estimates of mortality at 15 days were 0% with isothymol and 4% with placebo. No serious adverse events were reported in patients in the isothymol group who underwent randomization and in 13 of the 300 patients in the placebo group who underwent randomization (4.33%). Ex vivo analysis of blood plasma shows a close relationship between hypersensitivity of inflammatory mediators (macrophages and type I interferons) and significantly elevated histamine production, as evidenced by autophosphorylation and increased IL-6 production in blood monocytes of patients with severe COVID-19 compared to blood monocytes from healthy volunteers.
Conclusion: Isothymol was superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 and evidence of lower respiratory tract infection.
Isothymol; Carvacrol; SARS-CoV-2; COVID-19; Antiviral; RNA
Coronavirus 2019 is a new pandemic disease caused by a singlestranded RNA zoonotic virus called severe acute respiratory syndrome coronavirus 2 (Figure 1). The spectrum of COVID-19 ranges from mild respiratory illness to severe illness requiring hospitalization in up to a third of patients, with frequent progression to acute respiratory distress syndrome and high mortality [1].
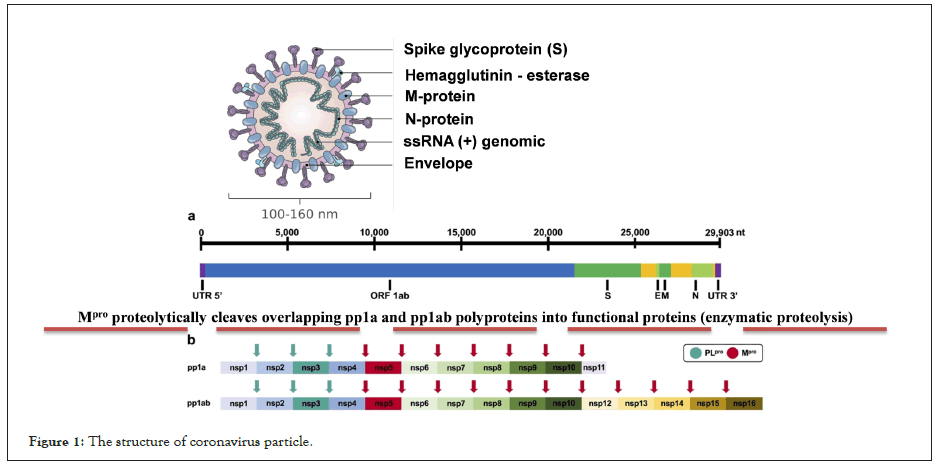
Figure 1: The structure of coronavirus particle.
The World Health Organization (WHO) in 2020, through its Director General Dr. Tedros Adhanom, declared the disease COVID-19 (produced by the agent SARS-CoV-2) as a pandemic, and at the date of writing of this final report (January 5, 2021), according to official data there are: 86 million infected people, +1.86 million deaths and fortunately +48.4 million people recovered, the countries with the greatest impact being: USA, India, Brazil, Peru, Russia, United Kingdom and Spain. Currently, the epicenter of the pandemic lies in America, given the increase in cases in the US, Brazil, Mexico, Colombia, Argentina and Peru.
The WHO declared to the international community in the months of June and July 2020 that they were evaluating +100 therapeutic treatments and possible vaccines that were in the testing phase in humans (Phase III and IV) (Figure 1).
This virus contains a genetic material of single-stranded RNA of positive (+) polarity, with a size of 27-32 kilobases (≈30,000 nucleotides (29,903), the size of the virions is approximately 50 to 200 nm in diameter). It is made up of a nucleocapsid and this in turn is made up of the RNA (+) and the phosphoprotein; this structure is covered with a lipid bilayer. Other structural proteins of the coronavirus are found here, such as the Spike protein that covers this viral particle, as well as Hemagglutinin-Esterase (HE) dimers; it also consists of the highly hydrophobic Envelope protein (E) and the Membrane protein (M), the most abundant on the surface of the virion. (2) Schematic representation of SARS-CoV-2 polyprotein cleavage sites. The papain-like protease PLpro cleaves at 3 distinct sites. The main protease Mpro (known as 3CLpro) cleaves at 11 distinct sites. The enzyme Mpro proteolytically cleaves the overlapping pp1a and pp1ab polyproteins into functional proteins. Essential replication enzymes such as RdRp or nsp13 cannot fully function without prior proteolytic release, thus Mpro is a key enzyme in the viral replication cycle. Consequently, its inhibition can stop the production of infectious viral particles and thus alleviate the symptoms of COVID-19 disease [2].
It has been reported that patients with COVID-19 may have a deteriorating biphasic clinical course after initial improvement, consistent with delayed and exaggerated immune activation. Producing a virus-induced hyperinflammatory response or "cytokine storm", which has been hypothesized as a main pathogenic mechanism of ARDS in these patients through the modulation of pulmonary macrophages, dendritic cells and/or neutrophils. The cell becomes stressed and undergoes a cytopathic effect (biochemical, molecular and cell viability changes caused during the viral cycle), and a cascade of inflammatory mediators begins to occur that will try to destroy the virus in the organism (Figure 2) [3].
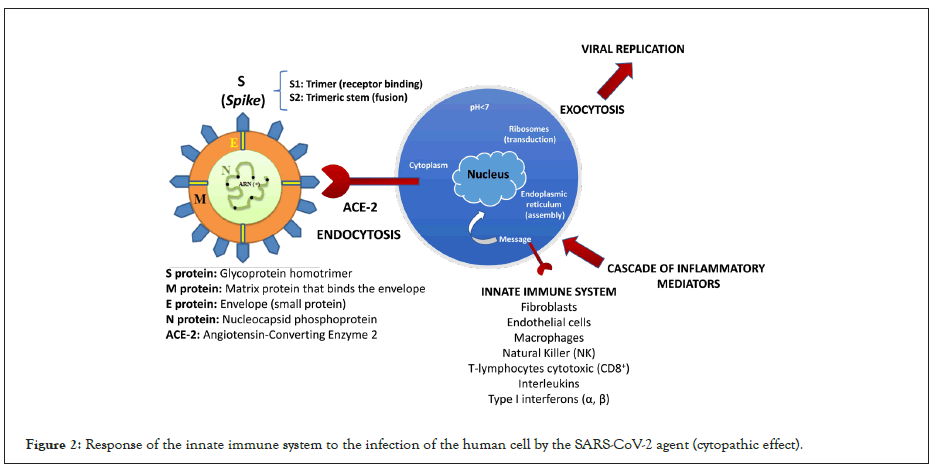
Figure 2: Response of the innate immune system to the infection of the human cell by the SARS-CoV-2 agent (cytopathic effect).
COVID-19 patients have elevated blood levels of multiple inflammatory cytokines and chemokines [interleukin-1β (IL-1β), IL-6, IL-7, IL-8, IL-9, IL-10, colony-stimulating factor granulocytemacrophage colony-stimulating factor, interferon-γ (IFN-γ), IFN- γ-inducible protein 10, monocyte chemoattractant protein 1, and macrophage inflammatory protein-1α], and those requiring admission to an intensive care unit have even higher levels of many of these proteins (Figure 2).
The hyper-inflammatory response in COVID-19 shares biological features with macrophage activation syndrome, suggesting that targeting the innate immune system may be an effective strategy [4].
The binding between the virus [SARS-CoV-2] and the host cell surface receptor known as Angiotensin Converting Enzyme 2 (ACE2) is formed by strong covalent bonds, predominantly between the amino acid histidine [C6H9N3O2] and the amino acid of the serine virus genome [C3H7NO3] present in the RBD domain (subunit 1 of the spike protein “S”). There is a catalysis between N and H+. The predominant amino acid [C6H9N3O2] in the ECE2 CINC sequence, referred to as carboxypeptidase [His-Glu-Xaa-Xaa-His], undergoes catalysis by decarboxylation [RCOOH] in the molecular structure, producing a hydrophilic amine [C5H9N3]. It is suggested that histamine (C5H9N3) intervenes in the hyperinflammation of the immune system by activating intracellular pathways that induce the production of cytokines in the lungs (cytokine storm) in the face of white cell infection, which is why the oxidation reaction of the amine by means of modified isothymol (Figures 2 and 3). In macrophages, Toll-like receptors (TLRs) recognize ssRNA from viruses such as SARS-CoV-2 and initiate signaling through histamine-dependent activation of nuclear factor κB (NF-κB), triggering the production of multiple inflammatory cytokines and chemokines and phagocytosis (Figure 2). It was shown that suppressing the production and adhesion of superoxide in neutrophils (immunostimulatory effect) through the regulation of cytokines and IL-4, IL-6 (interleukins) achieves the inhibition of the cytokine storm (phase 2B, infected patient). Furthermore, in a mouse influenza model, histamine inhibition decreased inflammatory mediators and rescued mice from lethal acute lung injury, suggesting that it may mitigate virus-induced lung damage caused by excessive inflammation [5,6].
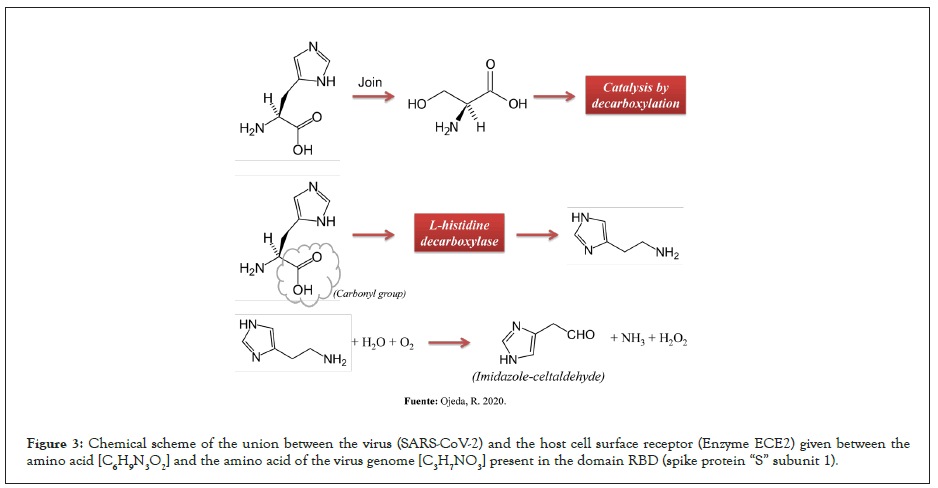
Figure 3: Chemical scheme of the union between the virus (SARS-CoV-2) and the host cell surface receptor (Enzyme ECE2) given between the amino acid [C6H9N3O2] and the amino acid of the virus genome [C3H7NO3] present in the domain RBD (spike protein “S” subunit 1).
Patient selection
A list of selection criteria was developed to identify patients who would potentially benefit from the use of isothymol to block excessive host inflammatory response and improve clinical outcome. Selection criteria included hospitalized patients with confirmed COVID-19 and hypoxia [room air blood oxygen saturation (SpO2) of 93% or less] requiring supplemental oxygen and ferritin ≥ 500 mg/mL, CRP ≥ 10 mg/dL, and/ or an ALC<1000 cells/μl. Treated patients ≥ 18 years of age, were able to take the drug orally (sublingually), and were not pregnant or lactating. Physicians at five hospitals were contacted to identify hospitalized patients who met these criteria and individual case-based discussions were held with treating physicians about the use of isothymol as an off-label treatment for patients who were undergoing treatment. Deteriorating or not improving in best supportive care.
Centers in which the trial was carried out
• Hospital Dr. Leopoldo Manrique Terrero-Periférico de Coche.
• Hospital Militar “Dr. Vicente Salias Sanoja”.
• Hospital Intermedio de Campaña “El Poliedro”.
Sponsor: Instituto Venezolano de Investigaciones Científicas (IVIC), Miranda, Venezuela.
Calendar and end date of the trial
Duration of the inclusion period: June 2020.
End date of the study: November 2020.
Treatment duration: 15 continuous days.
Isothymol treatment: Patients received the approved dose of isothymol 6 mg/mL orally (sublingually) every 4 hours for a median of seven days (patients on supplemental oxygen) and fifteen days (patients on invasive mechanical ventilation). Isothymol was recommended to be discontinued in patients who developed significant drug-related toxicity, which was not observed. Guidance was provided regarding the safe preparation of isothymol solution for patients who required an enteral feeding tube.
Pharmaceutical form isothymol: Each ml contains 6 mg of modified 2-Methyl-5-(1-methylethyl)-phenol (1% v/v). It is a light to medium yellow dilute lipophilic aqueous solution. Excipients s.q: cis-9-octadecenoic acid and Squalene (99% v/v). Carvativir® (Modified isothymol) was administered in a 15 cc presentation in an aseptic plastic container, labeled and sealed.
Evaluation and monitoring study: Local institutional practice guidelines were followed regarding the indications for supplemental oxygen supply, the need for mechanical ventilation, and laboratory studies of complete blood counts with differential cell counts and full chemistry panels. The oxygen saturation/fraction of inspired oxygen ratio was used to monitor daily changes in the patient's oxygenation status. To monitor for signs of inflammation, frequent monitoring of CRP, ferritin, fibrinogen, D-dimer, and IL-6 levels, which are non-experimental tests, was recommended whenever possible. All other studies were according to local doctors.
Ethical considerations: This trial was approved in August 2020, by the Bioethics Committee belonging to the Hospital Dr. Leopoldo Manrique Terrero-Periférico de Coche and was accompanied by the “Rafael Rangel” National Institute of Hygiene, both located in the city of Caracas in the Bolivarian Republic of Venezuela. In turn, each individual hospital consulted with local institutional review boards (IRBs) to ensure that the use of isothymol was ethically justified for the clinical situation. A document published by the WHO in 2006 addressing the use of unproven interventions during infectious disease outbreaks provides ethical guidance for the use of off-label medicines during a global pandemic. In the context of an outbreak characterized by high mortality, it is considered ethical to offer patients emergency experimental interventions and outside of clinical trials provided that: (i) There are no proven effective treatments; (ii) it is not possible to start clinical studies immediately; (iii) preliminary data exists to support the off-label use of a drug; (iv) the risk-benefit ratio for the patient is favorable; (v) qualified scientific advisory committee has approved the use of the drug; (vi) informed consent is obtained from the patient; and (vii) treatment results are documented and shared with the scientific community in a timely manner.
Informed consent: Each patient or their legally authorized representative received oral informed consent from staff experienced with isothymol at each hospital, which included a discussion of the risk and benefit of treatment, and was documented in the medical record (documented in the technical sheet of the drug). It was explained that the labeled use of isothymol to block the host's excessive inflammatory response in viral pneumonia (SARS-CoV-2 agent) had already been tested in previous clinical trials (phase I/II). The clinical experience with isothymol and its known safety profile (non-toxic and innocuous) were also discussed. The treating physician was included in these discussions to inform other treatment options for COVID-19 (stage I, II, and III patients). On a case-bycase basis, the risks/benefits were explained to the patient or their legally authorized representative so that they are aware of all possible treatment alternatives during their severe illness with COVID-19. It was explained that the risk of adverse events associated with 10 to 15 days of treatment was very low, and no serious adverse effects had been reported to date (clinical phase I/II). The patients who participated in the clinical study accepted the approved clinical protocol and gave their informed consent in accordance with the Declaration of Helsinki.
Clinical study participants: Blood samples were collected from patients in the morning and processed for flow cytometry-based analyzes of histamine and IL-6 phosphorylation. Plasma samples were shipped and analyzed at Mayo Clinic in September and November 2020. All study participants provided informed consent in accordance with the Declaration of Helsinki. Hospitalized patients were randomly located in different medical centers.
Design: Randomized, double-blind, multicenter study with blind evaluation of events, designed to compare the efficacy and safety of modified isothymol with placebo in patients ≥ 18 years of age who met the inclusion criteria. Treatment assignment was performed randomly in a 1:1 ratio to each of the two treatment groups defined according to their order of inclusion in the study. A master randomization list was generated for each group using a validated computer program using a random number generator. Randomization included patients who received modified isothymol and patients with placebo (control group).
Randomization was stratified by study site and disease severity at enrollment. Patients were considered to have severe disease if they required mechanical ventilation, required supplemental oxygen, chest pain, or another symptom consistent with bilateral atypical pneumonia, with one of the following paraclinical and imaging abnormalities: Oxygen saturation (SpO2) ≤ 93%, D-dimer elevation ≥ 10 mg/mL, Ferritin elevation ≥ 120 ng/mL, Fibrinogen elevation ≥ 400 mg/dL, Immunoglobulin M (IgM) elevation ≥ 200 mg/dL, Interleukin 6 elevation (IL-6) ≥ 1800 pg/mL and AP chest X-ray and chest computed tomography (CAT) showing thickening of the bronchi, consolidation and ground glass opacities [7]. Isothymol was administered orally (sublingually) at a dose of 6 mg/mL from day 1 until hospital discharge or death. A matching placebo was administered according to the same schedule and in the same volume as the active drug. A normal placebo lipophilic solution was used in the enrolled patients and to guarantee the individual opening of the randomization code, sealed envelopes were prepared for each subject, which will contain the specific treatment received by each one. All patients received supportive care according to the standard of care of the hospital where the trial was conducted. If a hospital had a written policy or guideline for the use of other treatments for COVID-19, patients could receive those treatments. In the absence of this policy, other experimental treatments or off-label use of marketed drugs intended to be a specific treatment for COVID-19 were prohibited from day 1 through day 15 (although such drugs could have been used earlier).
The trial protocol was approved by the institutional review board of the host hospital and was overseen by an independent data and safety monitoring board. Informed consent was obtained from each patient or from the patient's legally authorized representative if the patient was unable to consent.
Procedures: The patients were evaluated daily during their hospitalization, from day 1 to day 15. The clinical and paraclinical status of the patients was evaluated on an ordinal scale of eight conditions (defined below) and the national early warning score (which includes six physiological measures; total scores range from 0 to 20, with higher scores indicating greater clinical risk) were recorded each day. All serious adverse events and grade 3 or 4 adverse reactions that represented an increase in severity from day 1 and any grade 2 or higher suspected drug-related hypersensitivity were recorded and reported to the National Institute of Hygiene. "Rafael Rangel", using the form: "F-RCDM-014: Report of National Serious Adverse Events in Clinical Trials of Pharmaceutical Specialties". See the full description of the trial procedures in the Adverse Events section of the clinical protocol.
Statistical analysis: Statistical analysis was performed according to the "superiority" principle, which allowed detecting a significant difference between drug and placebo after parallel administration. A descriptive analysis of all the variables collected (variables related to efficacy and safety and all variables that may be considered related to the above) was performed. This descriptive analysis was performed for both treatment groups. Categorical variables were expressed as percentages and the number of observations. Continuous variables were expressed as mean and standard deviation or median and 25th and 75th percentiles, minimum and maximum. The variables that develop over time were also presented using Kaplan-Meier curves. Full details of trial design, conduct, monitoring, and analyzes can be found in the protocol and statistical analysis plan.
The main analysis was a stratified log-rank test of recovery time with isothymol compared with placebo, stratified by disease severity.
The primary outcome measure was time to recovery, defined as the first day, on which a patient met clinical conditions on the four-group ordinal scale. The sample was stratified according to the risk of the disease into 4 risk groups from highest to lowest: group 1: diagnosed patients and performance of procedures that generate aerosols (tracheal intubation, broncho-alveolar lavage or manual ventilation), group 2: diagnosed patients, but without performing procedures that generate aerosols, group 3: Undiagnosed patients, but with symptoms compatible with infection and group 4: asymptomatic undiagnosed patients. The clinical conditions of the four established groups are as follows: 1, not hospitalized, without activity limitations; 2, not hospitalized, activity limitation, home oxygen requirement, or both; 3, hospitalized, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalization was extended for infection control reasons); 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (medical conditions related to COVID-19 or others); 5, hospitalized, requiring supplemental oxygen; 6, hospitalized, requiring noninvasive ventilation or the use of high-flow oxygen devices; 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 8, death. Prespecified subgroups in these analyzes were defined by sex, disease severity (as defined by stratification and ordinal scale at enrollment), age (18 to 39 years, 40 to 64 years, or 65 years of age or older) and duration of symptoms after randomization (≤ 3 days or >3 days). Full details of trial design, conduct, monitoring, and analyzes can be found in the protocol and statistical analysis plan.
Statistical associations between time, SpO2/FiO2, CRP, ALC, and log IL-6 concentration were modeled as a mixed effect linear regression, with time points for each treated patient as independent observations, and with estimates of intercept and slope per patient. The model was fitted using the lmer function from the lme4 package. P values were calculated using a Wald test and are two-sided. Comparisons of the frequency of IL-6+, CD14+ monocytes or B cells under unstimulated or stimulated conditions and of histamine MFI in CD14++ monocytes or B cells between moderate to severe COVID-19 patients and healthy volunteers was performed using a T-test for unpaired data or, alternatively, a Mann-Whitney test, as the case may be, using GraphPad Prism 8.0, and presented as mean ± SEM.
Patients
This clinical study includes 600 hospitalized patients with COVID-19 who received modified isothymol and placebo between September 21, 2020 (treatment date of the first patient) and October 05, 2020 with formal data collection completed on November 30 of 2020.
A total of 300 patients in the isothymol group and 300 in the placebo group completed the trial through day 15. Subsequently, a total of 13 of the patients who were in the mild to moderate stratum at the time of randomization would meet the criteria for severe disease, resulting in 360 patients in the mild-to-moderate disease stratum and 240 in the severe stratum. No patient waived informed consent.
The mean age of the patients with isothymol was 56 years and 50.3% were men (Table 1). Based on the epidemiological evolution of COVID-19 during the trial, 79.8% of the patients belonged to health centers in Caracas, 15.3% in Miranda, and 4.9% in La Guaira. Overall, 53.3% of patients were white, 31.3% were of African descent, and 12.7% were designated as other or not reported. Most patients had two or more of the prespecified coexisting conditions at the time of acceptance to participate in the trial, most commonly hypertension (~60%), obesity (~17%), and type 2 diabetes mellitus (~13%).
| Isothymol | Placebo | |
|---|---|---|
| (ɳ=300) | (ɳ=300) | |
| Male/Female | 15/149 | 142/158 |
| Ages (media) | 18-94(56) | 18-85(52) |
| Days with symptoms | 5 | 10 |
| Treatment days | 15 | 15 |
| Number of patients requiring intubation during treatment | - | 31(10%) |
| Extubated patients | 39(13%) | - |
| Comorbid conditions | ||
| Hypertension | 189(63%) | 175(58%) |
| Diabetes myelitis type 2 | 35(12%) | 44(15%) |
| Morbid obesity | 45(15%) | 56(19%) |
| Apnea | 16(5%) | 23(8%) |
| Asthma | 65(22%) | 79(26%) |
| Rheumatoid arthritis | 18(6%) | 7(2%) |
| Leukemia | 12(4%) | 3(1%) |
| Prostate cancer | 6(2%) | — |
| Heart disease | 36(12%) | 29(10%) |
| Parkinson | 11(4%) | 9(3%) |
| Kidney failure | 17(6%) | 13(4%) |
| Signs and symptoms | ||
| Fever | 284(95%) | 268(89%) |
| Headache | 261(87%) | 281(94%) |
| Dyspnea | 85(28%) | 56(19%) |
| Diarrhea | 67(22%) | 43(14%) |
| Threw up | 47(16%) | 32(11%) |
| Abdominal pain | 39(13%) | 29(10%) |
| Loss of smell (anosmia and/or hyposmia) | 259(86%) | 273(91%) |
| Laboratory values | ||
| Absolute lymphocyte count (Cells/μL) | ||
| <1000 | 248(83%) | 229(76%) |
| >1000 | 52(17%) | 71(24%) |
| C-reactive protein (mg/dl) | ||
| ≥ 10 | 242(81%) | 218(73%) |
| Entre 3 y 10 | 55(18%) | 82(27%) |
| <3 | 3(1%) | - |
| Ferritin (ng/ml) | ||
| ≥ 500 | 264(88%) | 236(79%) |
| <500 | 36(12%) | 64(21%) |
| Fibrinogen | ||
| ≥ 400 | 235(78%) | 199(66%) |
| <400 | 65(22%) | 101(34%) |
| D-dimer | ||
| ≥ 0,5 | 221(74%) | 273(91%) |
| <0,5 | 79(26%) | 27(9%) |
| IL-6 (pg/ml) | ||
| ≥ 15 | 233(78%) | 259(86%) |
| <15 | 67(22%) | 41(14%) |
Table 1: Characteristics of symptomatic patients in phase I, II and III (ɳ=600).
Entry criteria for this study were confirmed COVID-19 virus requiring hospitalization for hypoxemia [blood oxygen saturation (SpO2) 93% or less] and evidence of inflammation [C-reactive protein (CRP) >12 mg/dl and/or ferritin >500 ng/ml] and/or lymphopenia [absolute lymphocyte count (ALC) <1000 cells/μl]. Among these symptomatic patients treated with isothymol, 151 (50.3%) were men, and the median age was 56 years with a range of 18 to 94 years (Table 1). One hundred thirteen patients received supplemental oxygen for a median of 3 days (range, 1 to 15), 75 of 300 (25%) of whom were on high-flow nasal cannula at the time they started modified isothymol (group with supplemental oxygen). All but three patients had increasing oxygen demand at the time of initiation of treatment. In addition, 48 patients were receiving invasive mechanical ventilation for a median of 6 (range, 1 to 21) days prior to isothymol administration (mechanical ventilation cohort). Coexisting medical conditions included hypertension in 189 of 300 (63%), obesity (body mass index >30 kg/m2) in 45 of 300 (15%), and diabetes mellitus in 35 of 300 (12%).
In the supplemental oxygen cohort (134/300), concomitant medications for the treatment of COVID-19 did not include steroids and/or hydroxychloroquine, and in the mechanical ventilation cohort no patients received an anti-IL-6 receptor monoclonal antibody or Remdesivir, only 6 mg/ml modified isothymol was administered every 4 hours for an average of 10 continuous days and dexamethasone. Laboratory evidence of inflammation with elevated CRP and/or ferritin was present in 297 of 300 (99% symptomatic patients). Patients had significantly elevated baseline laboratory abnormalities prior to the modified isothymol dose. Elevated CRP (>10 mg/dl) was present in 242 of 300 patients (81%), ferritin (>500 ng/ml) in 264 of 300 (88%), fibrinogen (>400 mg/dl) in 235 of 300 (78%), D-dimer (>0.5 μg/ mL) in 221 of 300 (74%), IL-6 (≥ 15 pg/mL) in 233 of 300 (78%), and severely decreased ALC (≤ 1000 cells/μl) in 248 of 300 (83%) (Table 1).
Regarding the patients treated with placebo, 142 (47.3%) were men, and the median age was 52 years with a range of 18 to 85 years (Table 1). One hundred nineteen patients received supplemental oxygen for a median of 9 days (range, 1 to 15), 90 of 300 (30%) of whom were on high-flow nasal cannula at the time they started placebo (oxygen group). supplementary). In addition, 27 patients were receiving invasive mechanical ventilation for a median of 11 (range, 1 to 21) days prior to placebo administration (mechanical ventilation cohort). Coexisting medical conditions included hypertension in 175 of 300 (58%), obesity (body mass index >30 kg/m2) in 56 of 300 (19%), and diabetes mellitus in 44 of 300 (15%). 10% of patients treated with placebo required intubation during treatment due to progressive decrease in blood oxygen saturation (SPO2 ≤ 80%), of which 9 patients died in the ICU.
Regarding the study of basal Histamine and hyperactivation of macrophages, an analysis of interleukin 6 (IL-6) was performed with COVID-19 patients with moderate to severe clinical condition (ɳ=150), and the results were compared with healthy volunteers (ɳ=130).
Given the pandemic emergency, the median number of days between symptom onset and randomization was 3 (range between grids, 3 to 12). A total of 493 patients (82.1%) had moderate to severe disease at enrollment; 75 patients (12.5%) met the criteria for category 7 on the ordinal scale, 165 (27.5%) for category 6, 253 (42.1%) for category 5, and 107 (17.8%) for category 4. During the study, 75 patients (12.5% of the 600 patients in the treated population) received a glucocorticoid (Table 2).
| Clinical condition of the patients according to the ordinal scale in the statistical model (1-8) | Total (ɳ) | Isothymol (ɳ) | Placebo (ɳ) |
|---|---|---|---|
| 4. Hospitalized, not requiring supplemental oxygen | 107 | 43 | 64 |
| 5. Hospitalized, requiring supplemental oxygen | 253 | 134 | 119 |
| 6. Hospitalized, requiring non-invasive ventilation or the use of high-flow oxygen devices | 165 | 75 | 90 |
| 7. Hospitalized, receiving invasive mechanical ventilation or Extra Corporeal Membrane Oxygenation (ECMO) | 75 | 48 | 27 |
| 8. Death | 12 | 0 | 12 |
Table 2: Clinical condition of COVID-19 patients according to the ordinal scale in the statistical model (1-8).
All patients (600) underwent chest X-ray and computed tomography (CT) at the time of confirmation of the disease and after treatment (days 1 and 15). CT was specifically indicated in patients with functional disorders, hypoxemia, or both after recovery from COVID-19.
CT scans performed in COVID-19 patients showed several changes in the lung parenchyma: ground-glass opacities: areas of lung opacity (very white) that does not hide underlying bronchial structures or blood vessels. Consolidation: an area of pulmonary opacity that hides the underlying bronchial structures and blood vessels Linear opacities, thickening of the bronchial wall. Inverted halo sign: an area of ground-glass opacity surrounded by a concentric ring of consolidation and disordered cobblestone pattern: presence of ground-glass opacity associated with thickening of the interlobular and intralobular septum (Figure 4 and Tables 3 and 4).
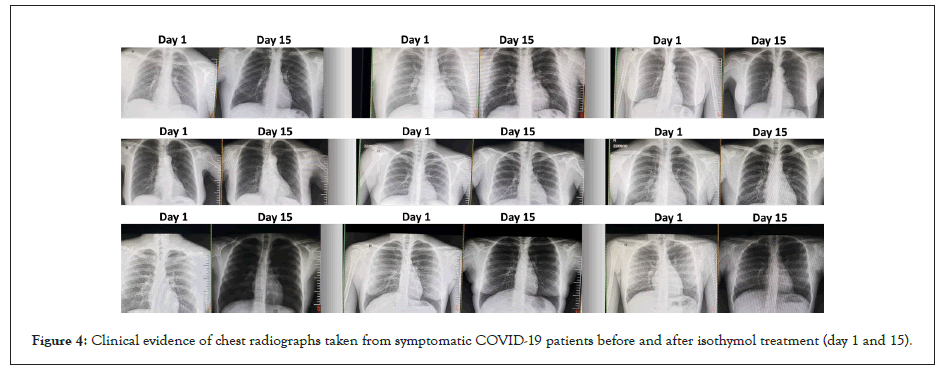
Figure 4: Clinical evidence of chest radiographs taken from symptomatic COVID-19 patients before and after isothymol treatment (day 1 and 15).
| CT scan characteristic | % |
|---|---|
| Ground Glass Opacity (GGO) | 54 |
| Pulmonary consolidation | 8 |
| GGO+consolidation | 14 |
| No GGO | 8 |
| Thickening of the bronchial wall | 16 |
Table 3: CT scan characteristics in treated COVID-19 patients.
| Tomographic characteristics of pulmonary opacities | % |
|---|---|
| Rounded | 42 |
| Linear | 9 |
| Messy cobblestone | 12 |
| Peripheral distribution | 15 |
| No axial distribution in the lung | 22 |
Table 4: Tomographic characteristics of lung opacities in treated COVID-19 patients.
Primary outcome
Patients in the isothymol group had a shorter recovery time than patients in the placebo group (median, 7 days compared with 14 days; recovery rate ratio, 1.24; 95% confidence interval [CI]) %, 0.78 to 1.87, P<0.001) (Figure 5 and Table 5). Recovery rate was higher among patients with a baseline ordinal score of 5 (recovery rate ratio, 1.57; 95% CI, 0.96 to 1.79); between patients with a baseline score of 4 and those with a baseline score of 6, rate ratio estimates for recovery were 1.28 (95% CI, 0.91 to 2.12) and 1.12 (95% CI, 0.87 to 1.76), respectively. For those receiving mechanical ventilation or ECMO at enrollment (initial ordinal score of 7), the recovery rate ratio was 1.02 (95% CI, 0.81 to 1.45).
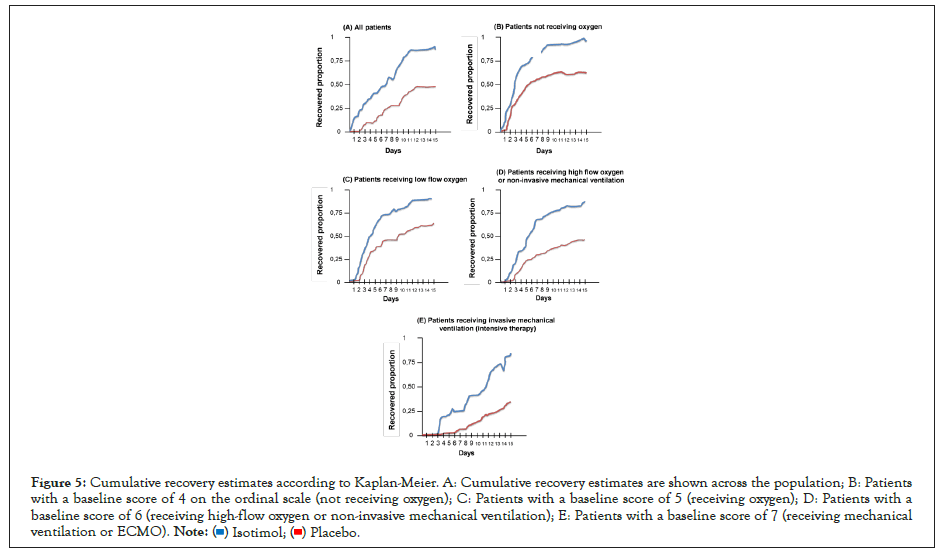
Figure 5: Cumulative recovery estimates according to Kaplan-Meier. A: Cumulative recovery estimates are shown across the population; B: Patients with a baseline score of 4 on the ordinal scale (not receiving oxygen); C: Patients with a baseline score of 5 (receiving oxygen); D: Patients with a baseline score of 6 (receiving high-flow oxygen or non-invasive mechanical ventilation); E: Patients with a baseline score of 7 (receiving mechanical ventilation or ECMO).
| Category | Total | 4 | 5 | 6 | 7 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | Isothymol | Placebo | Isothymol | Placebo | Isothymol | Placebo | Isothymol | Placebo | Isothymol | Placebo |
| Patient recovery | ||||||||||
| Number of recoveries | 276 | 146 | 43 | 37 | 125 | 63 | 68 | 38 | 40 | 8 |
| -92% | -49% | -100% | -58% | -93% | -53% | -90% | -42% | -84% | -30% | |
| Mean recovery time (95%CI) | 7 | 14 | 5 | 11 | 6 | 13 | 8 | 15 | 9 | >15 |
| Radius rates (95%CI), P<0.001 | 1,24 (0,78-1,87) | 1,28 (0,91-2,12) | 1,57 (0,96-1,79) | 1,12 (0,87-1,76) | 1,02 (0,81-1,45) | |||||
| Mortality † | ||||||||||
| Hazard ratios (95% CI) | 0,65 (0,37-1,01) | 0,37 (0,02-3,43) | 0,29 (0,04-0,87) | 0,97 (0,31-1,87) | 1,09 (0,57-2,01) | |||||
| Number of deaths (up to day 15) | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 9 |
| Kaplan–Meier estimate (95%CI) | 6,9 (4-8,3) | 13,9 (9,8-16,1) | 1,6 (0,1-8,2) | 2,7 | 2,1 (0,6-5,1) | 9,8 (9,3-18,7) | 11,3 (7,0-15,0) | 14,1 (10,7-27,9) | 11,5 (7,7-17,5) | 15,0 (11,2-29,3) |
Table 5: Kaplan-Meier estimates according to the ordinal scale score in the population with treated COVID-19 (statistical model).
Secondary outcome
The odds of improvement in ordinal scale score were greater in the isothymol group, as determined by a proportional odds model at the 15-day visit, than in the placebo group (92% of patients recovered on isothymol vs. 49% of patients recovered with placebo) (Table 5).
Mortality
Kaplan-Meier estimates of mortality at day 7 were 0% in the isothymol group and 1% in the placebo group 6 (hazard ratio, 0.97; 95% CI, 0.31 to 1; 87); estimates for day 15 were 2% in group 7 (1.09, 95% CI, 0.57 to 2.01). Between-group differences in mortality varied considerably by baseline severity (Table 2), with the largest difference seen among patients with a baseline ordinal score greater than 6.
Additional secondary outcomes
Patients in the isothymol group had a shorter time to improvement of one or two categories on the ordinal scale from baseline than patients in the placebo group (one category improvement: median, 5 vs. 11 days; rate ratio recovery, 1.28, 95% CI, 0.91 to 2.12 twocategory improvement: Median, 6 vs. 13 days, rate ratio, 1.57, 95% CI, 0.96 to 1.79) (Table 5). The initial length of hospital stay was shorter in the isothymol group than in the placebo group (median, 7 days vs. 14 days).
† Recovery rate ratios and hazard ratios were calculated from the stratified Cox regression model; P values for these ratios were calculated using the stratified log-rank test. Recovery ratio rates greater than 1 indicate a benefit for isothymol; hazard ratios less than 1 indicate a benefit for isothymol. Ordinal scale scores are as follows: 4, hospitalized, not requiring supplemental oxygen but requiring ongoing medical care (medical conditions related to COVID-19 or other); 5, hospitalized, requiring any supplemental oxygen; 6, hospitalized, requiring non-invasive ventilation or the use of high-flow oxygen devices; 7, hospitalized, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); and 8, death. Odds ratios and P values were calculated using a proportional odds model.
Oxygen requirements during treatment
To provide an estimate of a patient's oxygen requirement, given different supplemental oxygen flow rates and concentrations, the ratio of percent blood oxygen saturation to delivered oxygen concentration (SpO2/FiO2) was calculated with values higher representing improved oxygen uptake efficiency.
Measures of SpO2/FiO2 oxygen consumption requirement [% blood oxygen saturation (SpO2)/fraction of delivered oxygen (FiO2)] are displayed, a relationship accounting for both oxygen delivery and consumption (theoretical maximum 476 for 100% oxygen saturation in room air). Also shown are measures of inflammation (CRP, mg/dl) and ALC (cells/μl) at all available time points before and after isothymol treatment, which was started on day 1 (dashed line)
Among 134 patients in the supplemental oxygen cohort, the median duration of follow-up from the start of isothymol treatment was 5 days (range, 5 to 15). All patients received at least 15 days of isothymol, which was the expected duration of treatment. At the time of formal data collection (day 15), 100% of patients no longer required supplemental oxygen and had been discharged from the hospital.
Laboratory studies of inflammation and lymphopenia during treatment
Laboratory measures of inflammation were monitored during isothymol treatment (Figures 6 and 7). The earliest and most consistent indicator of decreased inflammation was the level of CRP. In the 134 patients with supplemental oxygen, CRP returned to normal in 134 (100%). Available serial IL-6 levels showed normalization in 85% of patients, and a 4-fold reduction in peak value in an additional 63 patients. Changes in D-dimer and fibrinogen were variable during the course of treatment and showed a favorable pattern in the recovery process. Similarly, ferritin was quite variable and fluctuated during the treatment period.
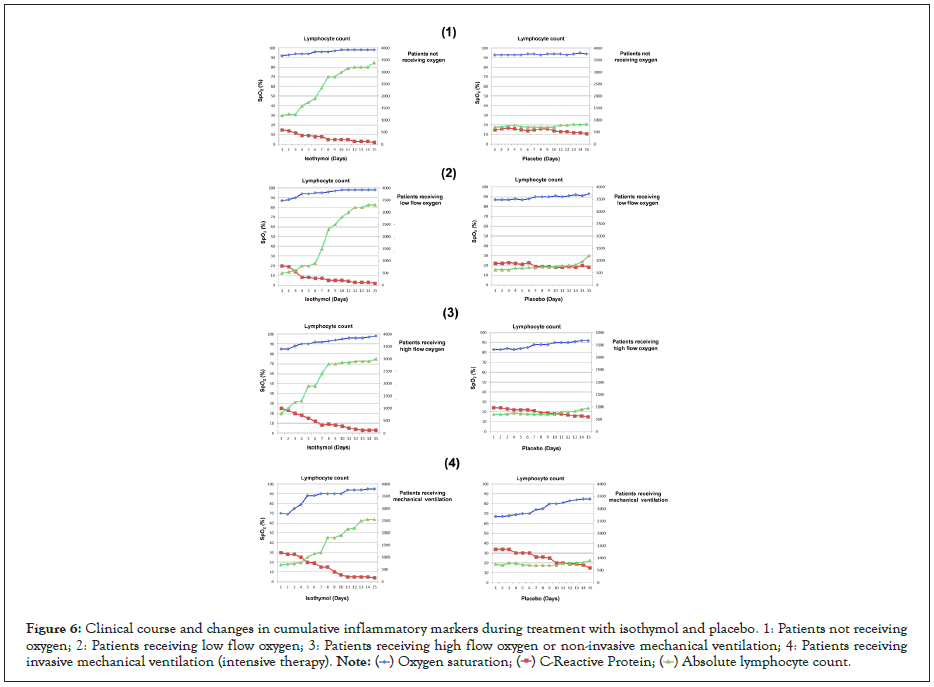
Figure 6: Clinical course and changes in cumulative inflammatory markers during treatment with isothymol and placebo. 1: Patients not receiving oxygen; 2: Patients receiving low flow oxygen; 3: Patients receiving high flow oxygen or non-invasive mechanical ventilation; 4: Patients receiving invasive mechanical ventilation (intensive therapy).
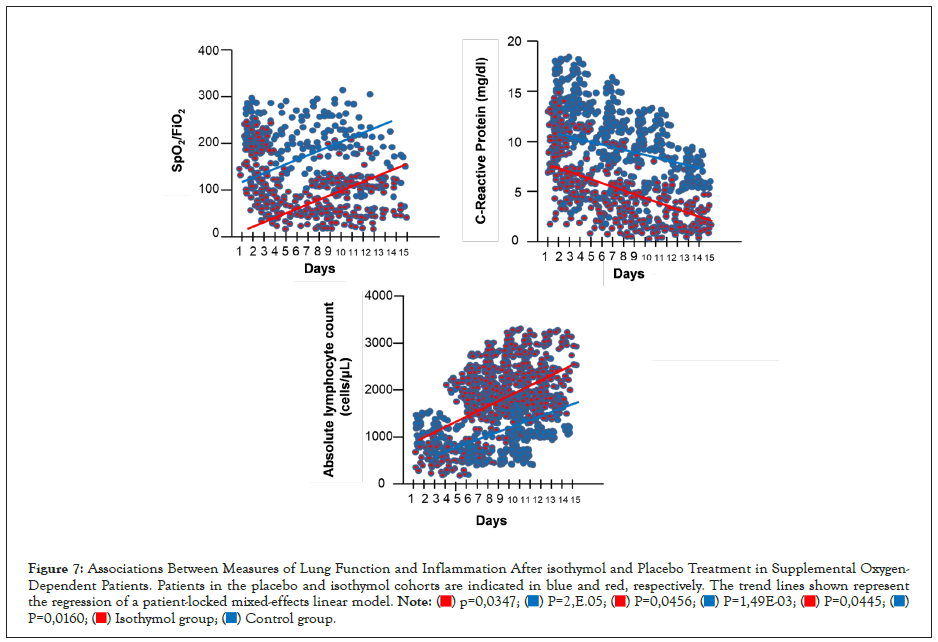
Figure 7: Associations Between Measures of Lung Function and Inflammation After isothymol and Placebo Treatment in Supplemental Oxygen-Dependent Patients. Patients in the placebo and isothymol cohorts are indicated in blue and red, respectively. The trend lines shown represent the regression of a patient-locked mixed-effects linear model.
The 48 patients who started isothymol while on mechanical ventilation showed a more variable and robust change in laboratory values compared to those on supplemental oxygen. CRP normalized in 39 patients (82%), all extubated in a variable period of 15 days. ALC values improved in most of these patients, with normalized values in 90% of patients.
In the case of patients with placebo, the variation in the CRP level was variable and a decrease <10 mg/dL was only achieved in 38% of the patients. ALC values remained <1000 Cells/μL in 63% of patients.
Correlation of CRP, ALC and oxygen consumption efficiency
Mixed-effects regression analysis showed that, over time, patients in the isothymol group generally increased their oxygen uptake efficiency (P=0.0347) and ALC (P=0.0445) and decreased their levels of CRP (P=0.0456), as illustrated by the trend lines in Figure 7. Distinct trends were observed in the placebo group with significant statistical representation. These results demonstrate a consistent association of the CRP and ALC biomarkers with clinical improvement as measured by oxygen uptake efficiency, both in the supplemental oxygen cohort and mechanical ventilation in the isothymol group.
Safety
No treatment-emergent toxicities attributable to isothymol were observed. No toxicities of special concern associated with isothymol, including cardiac arrhythmias, grade 3 or higher bleeding, diarrhea, allergies, interactions, and opportunistic infections were observed during the treatment period. For placebo, the most common non-serious adverse events occurring in at least 8% of all patients included: glomerular filtration rate decreased, hemoglobin level decreased, lymphocyte count decreased, respiratory failure, anemia, pyrexia, hyperglycemia, blood creatinine level increased and blood glucose level increased.
Histamine activation and IL-6 production in monocytes from patients with COVID-19
To examine whether isothymol target was activated in COVID-19 patients, basal histamine production was studied in whole blood samples from all moderate to severe COVID-19 patients and one hundred and thirty healthy volunteers (blood plasma). Significantly higher mean fluorescence intensity (MFI) of phosphorylated histamine was observed in CD14+ monocytes from patients with severe COVID-19 relative to that observed in healthy volunteers, an increase that was not due to differential levels of total histamine. IL-6 protein expression by immune cells in the blood of COVID-19 patients was then examined because production of this cytokine is known to be increased by histamine activity in normal human monocytes and macrophages.
Flow cytometry analysis of unstimulated whole blood samples revealed a significant increase in the percentage of IL-6+ CD14+ monocytes in patients with moderate to severe COVID-19 (ɳ=150) compared to healthy volunteers (ɳ=130). Treatment of these whole blood samples with the small molecule R848, a mimic of TLR7 and TLR8 activation by ssRNA, increased the percentage of IL-6+ blood monocytes, with levels significantly higher in samples from COVID-19 patients. compared to healthy controls.
The percentage of IL-6+ monocytes in patients with severe COVID-19 without ex vivo restimulation was comparable to that observed in monocytes from healthy volunteers after stimulation with R848. Histamine phosphorylation and IL-6 production were not observed in B cells in the same whole blood samples, demonstrating that histamine was specifically activated in monocytes from COVID-19 patients. Consistent with these findings, blood IL-6 levels in COVID-19 patients in this study significantly decreased during isothymol treatment (P=1.38 × 10−5). The IL-6 tests in blood plasma were carried out at the Mayo Clinic, during the month of September 2020.
Activation of immunogenicity
The primary outcome measure for immunogenicity was change from baseline in antigen—specific antibody levels—at 15 days (from day 1 to day 15), as measured by ELISA. Secondary immunogenicity outcomes were virus-neutralizing antibody titers (on days 7 and 15 after isothymol administration) and antigen-specific cellular immunity (specific T-cell immunity and interferon-interferon production). γ or lymphoproliferation) on days 7 and 15 during the administration of isothymol.
This double-blind, randomized, placebo-controlled trial identified an antiviral therapy as potentially beneficial in the treatment of COVID-19. These overall findings were consistent with the findings of the preliminary report: a 15-day course of isothymol was superior to placebo in the treatment of hospitalized patients with COVID-19. Patients who received isothymol had a shorter recovery time (the primary end point) than those who received placebo (median, 7 days vs. 14 days; recovery rate ratio, 1.24 [95% CI, 0. 78 to 1.87]). She is likely to have an improvement in ordinal scale score at day 15 (key secondary endpoint; odds ratio, 1.5; 95% CI, 1.2 to 1.9). All-cause mortality was 0% with isothymol and 4% with placebo (hazard ratio, 1.09; 95% CI, 0.57 to 2.01).
The data suggest that isothymol treatment may have prevented progression to more severe respiratory disease, as evidenced by the lower proportion of serious adverse events due to respiratory failure among patients in the isothymol group, as well as a lower incidence of new use of oxygen among patients who were not receiving oxygen at enrollment and a smaller proportion of patients who required higher levels of respiratory support during the study.
IIM exerts actions on other lineage cells via secondary messengers such as cytokines. Secreted miRNAs, as a form of exosomal miRNAs, are thought to be such secondary messengers.
Isothymol treatment was associated with fewer days of subsequent oxygen use for patients who received oxygen at enrollment and a shorter subsequent duration of mechanical ventilation or ECMO for those who received these interventions at enrollment. Taken together, these findings suggest that isothymol treatment may not only reduce the burden of disease, but may also decrease the use of scarce healthcare resources during this pandemic. Additionally, these studies suggest an important immunomodulation of IL-6 in critically ill patients (invasive mechanical ventilation) that allows the cytokine storm to be controlled by hyperactivation of macrophages.
The benefit of isothymol was most evident in patients with a baseline ordinal score of 6 (receiving high-flow oxygen). Part of this difference may be due to the larger sample size in this category, as the confidence intervals for baseline ordinal scores of 4 (receiving no oxygen), 6 (receiving high-flow oxygen), and 7 (receiving ECMO or ventilation mechanics) were extensive. Interaction tests suggest a greater benefit (with respect to recovery and mortality) in more median ordinal score categories.
On the other hand, clinical laboratory studies have revealed that histamine is a probable mediator of the pathological inflammatory response in severe COVID-19 (it induces cytokine storm by hyperactivation of macrophages). In accordance with World Health Organization (WHO) guidance, modified isothymol 1% v/v (in strict compliance with the Declaration of Helsinki) was prospectively administered with therapeutic intent to 300 hospitalized COVID-19 patients (symptomatic, in stage I, II and III). All symptomatic patients had increasing oxygen requirements at the time of initiation of treatment. The oxygenation and clinical status of most patients on supplemental oxygen improved relatively quickly after initiation of isothymol, which was temporarily associated with a normalization of inflammatory markers. Patients with mechanical ventilation had a more variable clinical response to isothymol, managing to improve ventilatory parameters, until extubation was achieved.
Laboratory studies of blood samples from hospitalized COVID-19 patients revealed significantly elevated histamine phosphorylation in peripheral blood monocytes compared to healthy volunteers, demonstrating that isothymol target is activated in these innate immune cells. This finding supports our view that the beneficial effect of isothymol in these patients was due in part to histamine inhibition at the target. More generally, this study highlights the opportunity to improve outcomes in severe COVID-19 patients by modulating the host inflammatory response, although most patients infected with SARS-CoV-2 have limited disease that does not require hospitalization, the patients in this series have progressed to a hyperinflammatory phase of this infection that can be fatal and for which there are no proven treatment strategies. All patients in our series had elevated inflammatory markers, including CRP, ferritin, and/or IL-6. Most of the patients also had increased levels of D-dimer, which may be associated with a coagulopathy that is common in COVID-19. Many patients in this series had severely depressed ALC, which has also been associated with severe COVID-19. Isothymol administration was temporally associated with a change in several of these inflammatory biomarkers, suggesting that histamine activation was triggering this pathology. In most patients, CRP levels normalized or decreased substantially, as did IL-6 levels. Similarly, lymphopenia rapidly normalized in most patients, possibly related to decreased inflammatory cytokines or chemokines. The inverse relationship between a measure of oxygen uptake efficiency (SpO2/FiO2) and CRP levels strongly suggested a link between improved lung function and decreased inflammation.
The apparent beneficial effect of Isothymol was clearly efficient between patients who received supplemental oxygen and those who required mechanical ventilation. In the supplemental oxygen cohort, oxygenation improved by 100%, with 90% discharged to room air despite pre-existing high oxygen requirements in the majority at three days of treatment. The benefit of Isothymol was quite marked in patients on ventilators, 81% were extubated after receiving isothymol. The association between oxygen uptake efficiency and CRP normalization was also evident in the mechanical ventilation cohort. These patients were clinically quite heterogeneous, including patients with significant organ dysfunction such as renal failure or who had been ventilated for a prolonged period prior to isothymol administration. Although the optimal time to start anti-inflammatory treatment was expected to be before deterioration requiring intubation, these results suggest that histamine inhibition may provide significant benefit to a subset of COVID-19 patients on ventilators.
Because the study investigated the effect of a limited course of COVID-19-positive patients treated with isothymol on all three phases of the disease, there was a need to know if the disease recurred after cessation of isothymol. Among the 209 patients who achieved room air status with isothymol, none had a recurrence, suggesting that a short course of isothymol was sufficient to calm the disease clinically.
The safety of any medication is always of paramount importance, but is further increased when used in an unproven disease state such as severe COVID-19 where multi-organ dysfunction occurs. It is within this context that isothymol was administered with careful consideration of its potential risks and benefits. The safety profile of isothymol is well defined in the context of long-term use over months or years in patients with various autoimmune pathologies and infections due to single-chain viruses and grams positive and negative bacteria. It is notable that no toxicity attributable to isothymol treatment has been observed, suggesting that, in the context of COVID-19, isothymol is very well tolerated, and could be defined as a GRAS (Generally Recognized As Safe) substance.
Ex vivo analysis of blood samples from moderate to severe COVID-19 patients revealed histamine activation in monocytes in all cases, as evidenced by significantly increased histamine phosphorylation compared to monocytes from healthy volunteers. Blood B cells had no evidence of histamine activation, suggesting that monocytes/macrophages may be the relevant in vivo target of isothymol in COVID-19. Consistent with this assay, IL-6 production was elevated in monocytes from COVID-19 patients, although there was no evidence of IL-6 production in B cells. Apparently, histamine was active in the entire population of COVID-19 blood monocytes, given the change of the entire histogram of histamine phosphorylation to higher levels. This finding is less likely to be attributable to trafficking of an activated monocyte subpopulation from the lung to the blood, but is more consistent with systemic activation of histamine in monocytes, whether by virus, viral RNA, or another circulating inflammatory mediator.
This widespread histamine activation in monocytes and macrophages argues that isothymol's clinical benefit derives from its ability to deactivate pathological histamine signaling in innate immune cells, which, in turn, extinguished the hyper-inflammatory process in these patients.
Isothymol may have been effective because it targets a source of cytokine production in innate immune cells rather than the downstream effector functions of individual cytokines. Other therapeutic strategies for COVID-19 have been considered, including corticosteroids. These agents provided little or no benefit in previous coronavirus epidemics and are not recommended for COVID-19. Only patients in our ordinal scale 7 (invasive mechanical ventilation) received steroid support. Other immune-modulatory strategies have been proposed, such as monoclonal antibodies directed at IL-6 or IL-1 receptors, which were not administered to patients in our series. Because multiple inflammatory cytokines and chemokines are elevated in COVID-19 patients, inhibition of any inflammatory mediator can only partially reduce the inflammatory process. Although histamine inhibitors interfere with B-cell activation and could lower antiviral antibody titers, this concern may be mitigated by timing of administration to patients with severe COVID-19, who are typically hospitalized 7 or more days later of the initial infection. A more complete understanding of how histamine inhibitors modulate the immune pathophysiology of COVID-19 will require the use of preclinical model systems in concert with detailed immune profiling of COVID-19 patients, before and during treatment with an inhibitor of inflammatory mediators. If immunomodulation of inflammatory mediators is of clinical benefit in moderate to severe COVID-19, as supported by these data, there is a need to know which histamine inhibitor would be optimal in this clinical setting given the association of COVID-19 with arrhythmias and other serious systemic sequelae of the inflammatory process. Isothymol also has detectable inhibitory activity against the immunologically important IL-2-inducible T-cell kinase (ITK) or against the epidermal growth factor receptor, a key signaling receptor on epithelial cells.
Clinical and laboratory findings in patients with COVID-19 are indicative of macrophage activation syndrome, which occurs in various clinical settings and is characterized by elevated CRP, IL-6, and other inflammatory cytokines, suggesting that the immunopathology of COVID-19 severe involves dysregulation of macrophage homeostasis. Consistent with this hypothesis, postmortem examination of COVID-19 lungs revealed a higher preponderance of monocyte/macrophage cells in the pulmonary alveoli. Histamine activation occurs in macrophages when TLRs bind to ssRNA, as can occur in SARS-CoV-2 infection, leading to NF-κB-dependent expression of multiple inflammatory cytokines and chemokines, including IL-6, it was observed that it was induced in COVID-19 monocytes and decreased in plasma after isothymol treatment. Histamine also regulates NLRP3 inflammasome formation in macrophages by physically associating with NLRP3 and phosphorylating its linker domain, triggering oligomerization and inflammasome formation. Histamine inhibition, either genetically or pharmacologically, markedly attenuates inflammasome formation in response to various stimuli. Although the model has been focused on macrophages, histamine is also known to control signaling in neutrophils, megakaryocytes, and platelets, which may also contribute to the immunopathology of severe COVID-19 and be kept in check by histamine inhibitors [8,9].
Several comorbidities associated with moderate to severe COVID-19 obesity, hypertension, atherosclerosis, and type 2 diabetes have been linked individually and as part of the metabolic syndrome to an elevated inflammatory state characterized by activation of the inflammasome in macrophages. These comorbidities could possibly set an elevated inflammatory "set point" that affects how macrophages respond to SARS-CoV-2 infections. This concept has been termed "trained immunity" or "innate immune memory" and results from epigenetic changes in gene expression in response to disease states or infections. Because infectious agents are powerful modifiers of innate immune memory, it will be important to assess whether SARS-CoV-2 infection exacerbates comorbid disease states and whether histamine inhibitors can prevent this.
Because of the histamine activation and IL-6 production that was detected in COVID-19 monocytes, histamine inhibitors (including isothymol administered in the trial) are proposed to target pathological monocyte/macrophage activation and dampen cytokine storm, which may consequently improve outcomes in these patients. More generally, the findings raise the possibility that morbidity from other disease states associated with macrophage activation, including severe influenza infections, may also be dependent on histamine function, also supporting evaluation of clinical trials. of histamine inhibitors in these clinical settings.
On the other hand, when analyzing the antigen-specific IgG, the seroconversion rate was 100% for the isothymol formulation at 15 days of the study, and when analyzing the neutralizing antibody responses, the seroconversion was 100% at day 15 of the study. Study for the formulation of isothymol (IgG ≥ 900 mg/dL). In the case of placebo, total IgG seroconversion was not evident at day 15 (≤ 300 mg/dL). Seroconversion rates on days 7 and 15 are presented in Figure 8.
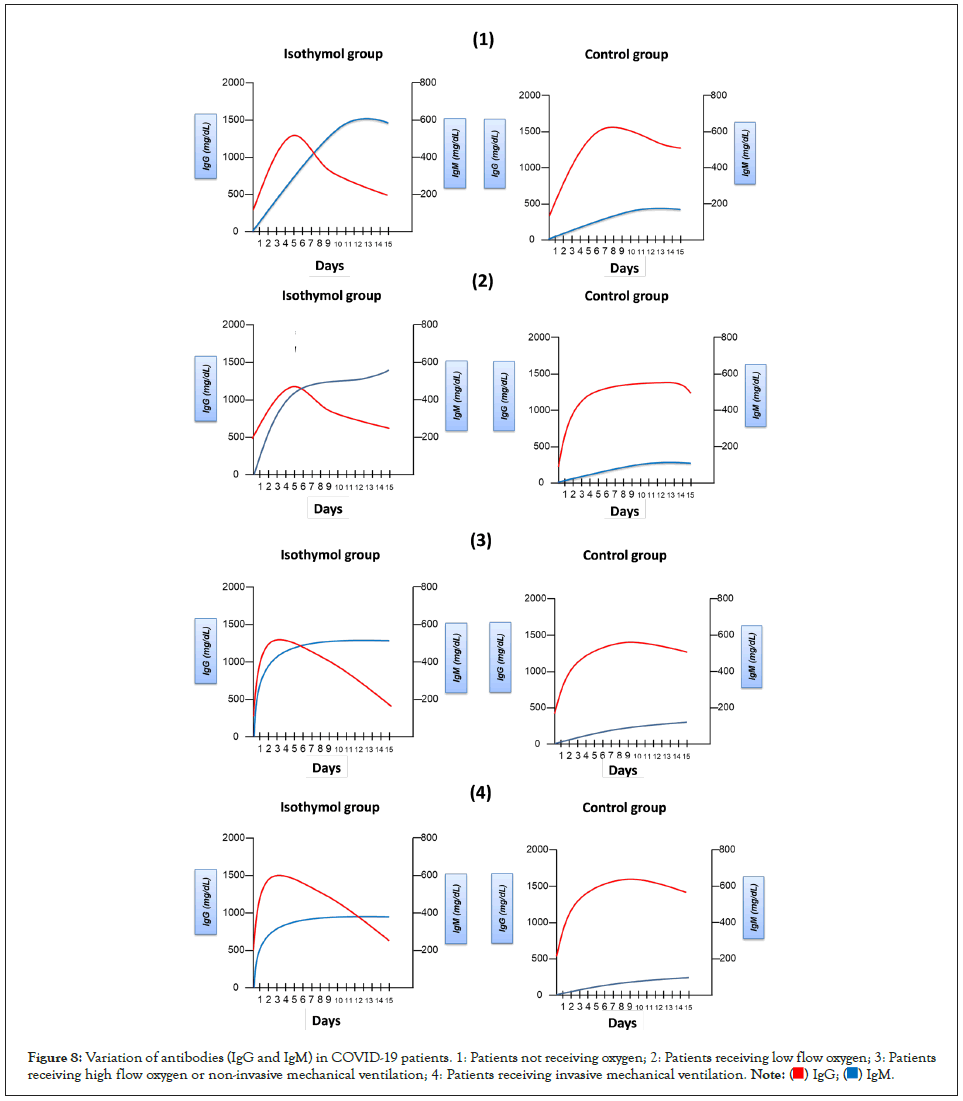
Figure 8: Variation of antibodies (IgG and IgM) in COVID-19 patients. 1: Patients not receiving oxygen; 2: Patients receiving low flow oxygen; 3: Patients receiving high flow oxygen or non-invasive mechanical ventilation; 4: Patients receiving invasive mechanical ventilation.
Cellular immune responses showed the formation of antigenspecific T-helper (CD4+) and T-killer (CD8+) cells, and an increase in the concentration of interferon-γ secretion in peripheral blood mononuclear cells, by 100% of the cases to which isothymol was administered. Cell-mediated responses were detected in all participants on day 15, with a mean cell proliferation of 4%-7% CD4+ and 3-6% CD8+ with the isothymol formulation [10-37].
An adjuvant called "squalene" is present in the isothymol formulation, under a stable emulsion with a surface-active substance (dispersion L/L), which could be the reason that explains the increased capacity of the antigen (immunogen) to induce a response immune (antibodies, specifically IgG). The action of squalene to prolong the exposure time of antigens to the immune system consists of improving antigen delivery to antigen-presenting cells, or providing immunostimulant signals that enhance the immune response. According to a report published by the WHO (2006), the safety of squalene was evaluated and they suggest that there are no problems in its administration in adult patients, infants and newborns (tested in influenza vaccines) (WHO, 2006).
This study was approved by the Ethics Committee in Research with Human Beings of the institution of origin (protocol LAB-2020-01) and registered in the ISRCTN Registry, number ISRCTN15363958 and ClinicalTrials.gov identifier, NCT number: NCT05445089.
Acknowledgements
This study was financed by the Venezuelan State through the Venezuelan Institute for Scientific Research (IVIC).
Both verbal and written consents were obtained from each patient and/or care giver before starting the study.
The detailed data that support the findings of our experimental study are available from the corresponding author upon reasonable request.
All the authors have contributed to, seen, and approved the final, submitted version of the manuscript.
The authors declare no competing interests.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Ojeda RA (2022) Clinical Study to Verify the Effectiveness and Safety of the Modified Isothymol or Carvacrol Compound against SARSCoV- 2 in COVID-19 Patients. J Clin Cell Immunol. 13:670.
Received: 09-Aug-2022, Manuscript No. JCCI-22-18750; Editor assigned: 12-Aug-2022, Pre QC No. JCCI-22-18750 (PQ); Reviewed: 26-Aug-2022, QC No. JCCI-22-18750; Revised: 30-Aug-2022, Manuscript No. JCCI-22-18750 (R); Published: 09-Sep-2022 , DOI: 10.35248/2155-9899.22.13.670
Copyright: © 2022 Ojeda RA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.