
Cloning & Transgenesis
Open Access
ISSN: 2168-9849

ISSN: 2168-9849
Research Article - (2022)Volume 10, Issue 1
Bacterial cell culture is superior to using a cheap culture medium and rapid cell growth at high concentrations. High concentrations of cells increase the rate of protein production in the bacterial expression system, resulting in high volumes of recombinant protein. E.coli is the most common host and the first choice for making many recombinant proteins. One of the most important substances in protein production is the expression carrier properties used. Having a good and adjustable promoter in the cloned gene is one of the most important control characteristics for the gene host.
Bacterial cell culture; High concentrations; Recombinant protein
Recombinant DNA technology, also called gene cloning or gene manipulation, plays an important role in the production and development of recombinant drug proteins as an achievement of genetic engineering. The four main stages of recombinant DNA technology are: obtaining the DNA fragments encoding the desired protein, attaching the foreign DNA fragment to a vector, transferring the resulting recombinant structure to the biological system, and screening the colonies receiving the recombinant structure.
Bacterial cell culture is superior to using a cheap culture medium and rapid cell growth at high concentrations. High concentrations of cells increase the rate of protein production in the bacterial expression system, resulting in high volumes of recombinant protein. E.coli is the most common host and the first choice for making many recombinant proteins. One of the most important substances in protein production is the expression carrier properties used. Having a good and adjustable promoter in the cloned gene is one of the most important control characteristics for the gene host.
One of the most important causes of death in the world today is impaired blood flow due to the formation of blood clots. Clot formation or thrombosis in the circulatory system causes blockage of arteries and can have dangerous consequences such as stroke and death. The fibrinolytic compounds available today activate the fibrinolysis system and have serine protease activity, and their mechanism of action is the conversion of plasminogen to plasmin, a natural fibrinolytic agent. Plasmin breaks down fibrinogen and fibrin in the clot to dissolve the clot. Plasminogen activators have both direct and indirect functions. Direct activators include altoplas, ratplas and urokinase, and indirect activators include streptokinase and staphylokinase.
Streptokinase is an extracellular protein, which is produced by different types of Beta hemolytic streptococci belonging to groups A, C and G. It is one of the drugs that can form complex complexes with plasminogen molecules one by one and cause the clot to dissolve. Streptokinase is marketed under the brand names Streptase, Kabinase, Bikinase, kinalysin, braun, awelysin. This drug is distributed in Iran under the brand name of Braun by Daru Company and is imported from Germany. Streptokinase, by binding to plasminogen, Asp41-His region 48, is involved in the identification and activation of plasminogen and C-terminal region 48, in the activation of the amino acid region of 59 plasminogen.
Due to the increasing production of recombinant drugs, steps should be taken to produce this drug because it causes self-sufficiency in drug production and independence from drug imports. Therefore, in this study, the cloning of streptokinase gene, under the heat-sensitive promoter of lambda phage, on the chromosome of E. coli has been investigated [1].
In this study, two types of culture medium: LB liquid culture medium and LBA solid culture medium were used for bacterial culture. For every 1 cc of culture medium, 1 μl of antibiotic with concentration was added to the medium. Extraction of DNA and RNA from bacterial cells was obtained by buffer containing Tries and EDTA. Plasmid extraction was then performed. Agarose gel was used to analyze DNA and RNA products and to examine DNA extracted by spectrophotometer. Thus, the amount of Optical Density (OD) of the solution was measured at 260 and 280 nm and first the OD280/OD260 ratio was used to calculate the purity of DNA. DNA extraction of E. coli strain HB101 was performed by phenol-chloroform method. Plasmid extraction from E. coli top 10 bacterial colonies was also performed by alkaline lysis method. DNA transfer to the bacterial cell was then performed.
Plasmids that were transformed in this study include: PTZ57R plasmid for T-vector preparation, pBluescript iisk plasmid (+/-) as the main vector for structural preparation, T vectors with ARA1 and ARA2 homologous arms, pgp 1-2 plasmid containing promoter The synthetic pgh plasmid contained the streptokinase gene with the cloned promoter and the pbluescriptiisk plasmid (+/-) contained two homologous arms with the streptokinase gene and the promoter. TA Cloning method was used for cloning the PCR product. PTZ57R and pbluescriptiisk (+/-) vectors containing lacz gene were used to establish white and blue colonies in this process. T vector used in this study was PTZ57R. In order to perform PCR of homologous arms, PCR reaction was first performed on the E. coli genome of HB101 strain, which was extracted by specific primers of homologous arms called ARA1 and ARA2. PCR of the first homologous arm (ARA1) was performed on the HB101 genome at four temperatures of 68, 65, 62, 60°C, in which 430 bp fragments was visible. The second homologous arm (ARA2) was performed at three temperatures of 64, 62, and 60°C, with a 435 bp fragment visible. PCR products were incubated at 22°C for 2 hours due to the addition of nucleotide A by the enzyme Taq polymerase at both ends, with a ligation with T vector and a vector of plasmid PTZ57R. The contents of the binding reaction were then transformed into the Top10. To confirm cloning, the plate resulting from the binding reaction contained two types of blue and white colonies, which were white colonies. After these colonies, PCR colony was first performed for both plots 1 and 2 by universal primer PTZ57R. Then the confirmed colonies were cultured in liquid medium with appropriate antibiotics and the next day plasmid was extracted and finally the plasmid was confirmed by universal primer and specific PCR [2]. The confirmed plasmids were transformed into TOP 10 bacteria, and plasmid was extracted for sub-cloning steps in the vector. Positive colonies were cultured in antibiotic medium and their plasmids were extracted. Plasmids extracted by PTZ57R universal primer and specific for the second homologous PCR arm were finally confirmed by fragment removal by two enzymes, KpnI, ApaI and clone II. Finally, confirmation of promoter function and expression of streptokinase protein at 42°C was performed by Western blotting.
After performing the steps mentioned in the research method, using SDS-PAGE technique, the expression of protein was qualitatively examined, which showed that the expression of protein with a molecular weight of 47 kDa increased at 42°C (Figure 1). Finally, Western blotting using monoclonal antibodies against streptokinase and secondary antibodies showed that the protein expression was correct.
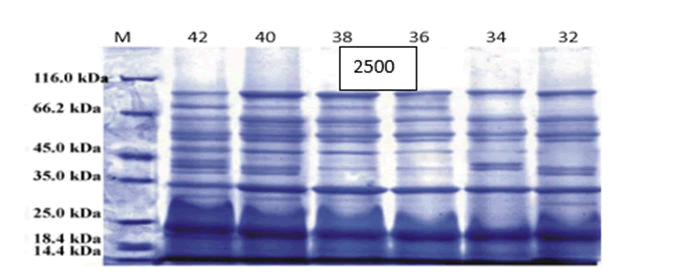
Figure 1: SDS-PAGE Protein expression is increased at 42°C.
Failure of blood to clot during hemorrhage or homeostasis can have dangerous consequences. Stopping blood flow by external blood clotting with the help of external factors can be done by fibrinolytics such as: urokinase, tissue plasminogen activator and streptokinase and save the person from the risk of death [3].
Streptococcus strains that are capable of producing streptokinase produce very low levels of blood coagulation activator, which is not capable of industrial production. This is why the industrial production of this valuable substance is difficult and attention has been focused on genetic engineering, and so far hosts such as Escherichia coli,Bacillus subtilis and Pichia pastoris have been used to express this recombinant protein. The choice of an optimal expression system depends on factors such as the properties of the recombinant protein itself as well as its possible use after production and must be able to produce acceptable amounts of protein. Over the last 30 years, many advances have been made in recombinant protein expression systems [4].
Escherichia coli have been widely used as an expression host to produce recombinant proteins. In this study, Escherichia coli were also used as a host, because this microorganism is well known and its different types have been developed for different research purposes. The promoter used in this study was a heat-sensitive Pl promoter from the lambda phage. One of the advantages of this promoter is that there is no need to use expression-inducing chemicals to turn on the promoter, and by simply changing a physical parameter, namely temperature, protein expression can be induced under this promoter [5,6].
The results of this study were the insertion of the gene responsible for the production of streptokinase in the genome of E. coli strain Top 10 with the help of ARA1 and ARA2 homologous arms and the expression of this protein under the lambda phage heat-sensitive promoter. The results of SDS-PAGE and Western blotting confirm the successful cloning and expression of this gene under a heat-sensitive promoter. As the temperature reached 42°C, the band observed in SDS-PAGR as well as the spot observed in Western blot showed higher protein expression.
In Used PCR to extract and amplify the streptokinase gene from Streptococcus dysgalactiae of the equisimilis strain, then transfected it into the PET 32a expression vector and cloned it into the E. coli BL21 (DE3) host. Gene expression was induced by the beta-galactosidase promoter by IPTG and the protein produced by this method was purified by chromatography with Ni-NTA resin. In this study, were able to achieve a yield of 470 mg of recombinant streptokinase per liter of culture medium. In a study by Similar to this study by cloning the streptokinase gene in Escherichia coli, the expression of this protein was higher at 42°C than at other temperatures tested. The SDS-PAGE results show this to be true and show hope for genetic engineering to increase the production efficiency of streptokinase as an economically valuable anticoagulant.
The difference between this research and previous studies is in the use of additional vector. This vector ensures the stable expression of the gene by transferring the desired gene into the main genome of the host bacterium. The use of a heat-sensitive promoter also eliminates the need to use chemicals to induce expression, as these chemicals themselves can act as growth-limiting agents in bacterial growth and have a negative effect on the production of recombinant proteins.
In Cloning and expression of streptokinase isolated from Iranian S. equismilis in Escherichia coli were performed. Their reports showed that the expression of recombinant streptokinase proteins with a molecular weight of 47 kDa was correctly performed in Escherichia coli, which was confirmed by Western blotting and SDS-PAGE techniques. The results obtained in Dr. Keramati's research are consistent with our results. Also cloned the genomic DNA of Streptococcus equisemilis into Escherichia coli using Lambda bacteriophage. Different plasmids were studied, but the most effective expression was PBR322.
[Crossref ] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref ] [Google Scholar] [Pubmed]
[Crossref ] [Google Scholar] [Pubmed]
[Crossref ] [Google Scholar] [Pubmed]
Citation: Yavar R (2022) Cloning of Streptokinase gene under thermal sensitive promoter of lambda phage into E. coli genome. Clon Transgene. 10:001
Received: 18-Feb-2022, Manuscript No. 15248; Editor assigned: 21-Feb-2022, Pre QC No. 15248; Reviewed: 07-Mar-2022, QC No. 15248; Revised: 11-Mar-2022, Manuscript No. 15248; Published: 18-Mar-2022
Copyright: © 2022 Yavar R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.