Clinical & Experimental Cardiology
Open Access
ISSN: 2155-9880
ISSN: 2155-9880
Research Article - (2024)Volume 15, Issue 5
Background: Coronary Artery Disease (CAD) remains a leading cause of mortality, with Indians exhibiting a notably elevated risk compared to other populations. Digital Therapeutics (DTx), such as smartphone applications, offer a promising solution by providing personalized, technology-driven interventions to improve healthcare outcomes.
Methods: This interim, prospective, single-centre cohort study aimed to evaluate the effectiveness of a clinical evidence-based software-driven therapeutic LYFE app intervention in combination with Standard of Care (SOC) compared to SOC alone among post-Percutaneous Coronary Intervention (PCI) patients with CAD and/or ACS.
Results: A total of 86 patients were enrolled, with 42 receiving the LYFE app intervention alongside SOC and 44 receiving SOC alone. At 3 months, the LYFE group experienced significantly greater improvements in mean Dartmouth COOP Scales compared to the SOC group (p<0.001). The mean change from baseline to 3 months in SBP was higher in the LYFE group compared to the SOC group (6.1 vs. 1.9). LYFE showed favourable trends in Low Density Lipoprotein (LDL) (66.5 mg/dL vs. 61.3 mg/dL) and glycated Haemoglobin (HbA1c) (7.6% vs. 6.9%) from baseline to 3 months; which attributed to improved adherence to medications and app assistance. At 3 months, participants in the LYFE group showed higher medication adherence, with 85.4% reporting 'hardly or no difficulty’ in remembering their medication compared to the SOC group (42.1%). Compared to the SOC group, the LYFE group showed superior adherence in terms of physical exercise (92.7% vs. 28.9%) and diet (95.1% vs. 50.0%) at 3-months.
Conclusion: The combination of LYFE app with SOC, significantly improved medication adherence, physical exercise adherence, and diet habits compared to SOC alone. It also led to notable enhancements in Quality of Life (QoL), reductions in SBP and PR, improved blood pressure control, and positive changes in biochemical parameters such as LDL cholesterol and HbA1c levels.
Acute coronary syndrome; Coronary artery disease; Software-driven digital therapeutics
Coronary Artery Disease (CAD) can be identified as the worldwide leading cause of mortality and loss of disability adjusted life years, characterized by the presence of atherosclerosis in the coronary arteries [1,2]. CAD is seen to develop in young Indian population, with >50% mortality seen in those <50 years of age [1]. The treatment goals in patients with CAD are alleviation of symptoms and prevention of probable Cardiovascular events (CV) like Myocardial Infarction (MI), stroke, and death [3]. The 2013 ESC guidelines recommends lifestyle adjustments, risk factor reduction, pharmacological treatments, and/or revascularization as integral components of CAD management [4].
Percutaneous Coronary Intervention (PCI) is a fundamental treatment in Acute Coronary Syndrome (ACS), followed by a rigorous antithrombotic regimen for improved prognosis in patients with ACS [5,6].
In spite of these multiple pharmacological advancements, cardiologists continue to grapple with various challenges, including limited diagnostic data, poor adherence rates, frequent hospital readmissions, the absence of standardized cardiac rehabilitation, personalized diet and exercise plans, tailored lifestyle guidance, inadequate patient awareness and education, a lack of understanding regarding the consequences of poor adherence on heart health, delayed seeking of counselling, and an elevated risk of hospitalizations [7]. Another challenge faced by the cardiologists is non-adherence to secondary prevention medications post ACS [8], which might stem out the elevated risk of recurrent hospitalizations. This is higher in the Indian population, particularly within the age group of <40 years of age [9].
Such drawbacks demand a meticulous approach that engages diligent medical or interventional strategies and management plans to enhance the mortality and morbidity outcomes. One such innovation is the emergence of Digital Therapeutics (DTx), which utilizes technology such as smartphones, wearable devices, and cloud-based platforms to improve the healthcare and wellness. Furthermore, it focuses on monitoring and encouraging positive behavioural changes by adopting approved therapeutic interventions like smartphone applications [10]. The DTx offers a tailored approach to patients catering to their clinical needs, goals, and lifestyle modifications by adapting a personalized and active management plan [11]. A randomized study has observed that long-term patient care with improved clinical outcomes care can be ensured more precisely through an app-based system than a traditional hospital-based follow-up protocol alone [12]. A review of literature by Phan, et al., has inferred that DTx either alone or in combination with the conventional pharmacological approaches, can enhance treatment adherence and efficacy in patients, it also suggests that the benefits conferred by DTx are highest when used in combination with pharmacological therapies [13].
In pursuit of the above context, the present study aims to evaluate the effectiveness of a clinical evidence-based software-driven therapeutic LYFE app intervention along with SOC compared to only SOC group among post-PCI patients.
Study design
This was an interim, prospective, single-centre, real-world cohort study conducted between Feb. 2023 and Sep. 2023. The study adhered to the Institutional Ethics Committee (IEC), Good Clinical Practice (GCP) guidelines, all relevant Health Authority requirements, and national laws.
Study population
Patients aged 18 years or older, diagnosed with CAD and/or ACS, who underwent PCI (emergency or elective), wiling to comply with the follow-up plan, has read and signed the informed consent form, possessing basic reading skills in English, Hindi, or Marathi were enrolled in this study. A total of 86 patients were included, with 42 patients in the LYFE group who received both software-based DTx with SOC and 44 patients in the SOC group who received SOC only.
Study procedure
A total of 86 patients were enrolled in this study; with 42 patients randomized into LYFE group whereas, 44 randomized into SOC group. At 1 month, the LYFE group reported one death resulting into 41 patients, whereas there was no change in SOC group. At 3 months, four patients were lost to follow-up and two deaths were reported in SOC group resulting in a total of 38 patients (Figure 1).
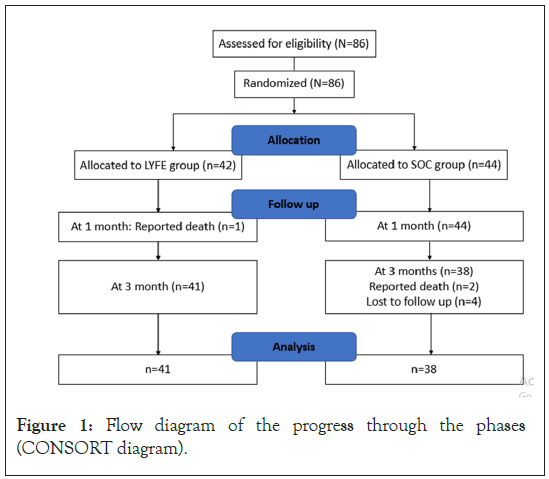
Figure 1: Flow diagram of the progress through the phases (CONSORT diagram).
Intervention
SOC: The SOC involved dual antiplatelet therapy, advising ACS patients to continue it regardless of stent type. Stable CAD patients were instructed to maintain dual antiplatelet therapy after bare-metal or drug-eluting stent implantation. Additionally, the standard care considered adding an oral anticoagulant to dual antiplatelet therapy for ACS cases, with decisions on the duration of dual antiplatelet therapy influenced by bleeding risk and participant-specific standard care.
LYFE (Leading Your Future Experience) app: LYFE app, a product of (Lupin digital health Pvt Ltd) is a personalised digital heart care program designed by cardiologist that allows patients to monitor and manage heart health, comprises of Mobile app integrated with connected devices (wireless activity and heart rate tracker, blood pressure monitor, pulse oximeter, glucometer, Smart weighing scale and ECG handheld). Integration of wireless devices allowed patients to measure and monitor their blood pressure, heart rate and physical activity. Patients received reminders on medications, lifestyle modifications and appointments. LYFE program has seven components: 1) Comprehensive and proactive monitoring with suite of auto-scheduled lab tests and teleconsultation with the treating cardiologists, 2) Adherence to lifestyle changes and medication through nudges, providing actionable, personalized insights and bite-sized competition, 3) Caregiver involvement through training and dedicated caregiver app to get alerts and monitor vitals, 4) Personalized coaching and support from dedicated nutritionists and health coaches to help patient manage disease through diet and exercise plan which is contextual to patient's lifestyle, condition and preference, 5) Education modules on disease for the patient and care giver, 6) Emergency response system to help patient manage any cardiac emergencies. It also includes access to early detection system (symptoms based/auto triggered SOS button on fall detection, erratic heart rate) that alerts doctors and family members and triggers emergency protocol, 7) Access to ambulances equipped to handle cardiac events and pre-determined hospitals based on availability and 1st aid education (Figure 2).
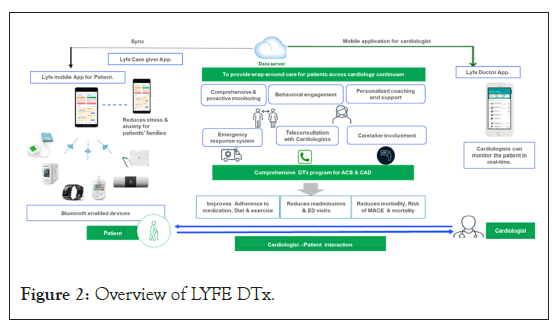
Figure 2: Overview of LYFE DTx.
Follow up
The patients were evaluated at 1 month and at 3 months by tracking changes in QoL using the Dartmouth COOP Questionnaire scores from baseline to the end of the study period. In addition, the assessment included changes in weight, BMI, vital signs such as BP, Pulse Rate (PR), laboratory parameters, including High- Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), Triglycerides (TG), Total Cholesterol (TC), Haemoglobin (Hb), Fasting Blood Glucose (FBG), Post-Prandial Blood Glucose (PPBG), Glycated Hemoglobulin (HbA1c) levels and creatinine.
Endpoints
The primary objective of this study was to evaluate the effectiveness of a clinical evidence-based software-driven therapeutic LYFE app intervention along with SOC compared to only SOC group among post-PCI patients with CAD and/ or ACS by assessing adherence to medication, physical exercise, and diet habits. Secondary objectives included the analysis of individual QoL components, monitoring hospital admissions, and scrutinizing Major Adverse Cardiovascular Events (MACE), such as cardiovascular death, nonfatal MI, stent thrombosis, revascularization, stroke or transient ischemic attack, and significant bleeding events.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). Descriptive statistics were used to perform a statistical analysis of the collected data using the mean and standard deviation for all datasets. To evaluate significant differences in mean changes between the baseline and follow-up periods, a paired and independent t-test was used. A p-value <0.05 was considered as statistically significant between the groups.
Demographic characteristics in patients
A total of 86 patients were included in this study. The mean age of the patients was higher in SOC group (56.5 years) compared to the LYFE group (51.8 years). The mean Body Mass Index (BMI) between both the groups was comparable (26.9 kg/m2 vs. 26.2 kg/ m2). The proportion of the male population was higher in both the groups when compared to female population (LYFE: 76.2% vs. 23.8%; SOC: 72.7% vs. 27.3%). Upon considering the personal habits, alcohol consumption was found to be significantly higher in SOC group (6.8%) when compared to LYFE group (26.2%), p=0.020. One of the prerequisite for this study was post- PCI patients, the history of PCI was higher in patients within SOC group (43.1%) than the LYFE group (33.3%). Among the comorbidities, although not statistically significant hypertension was observed to be higher in LYFE group (76.2%) than the SOC group (68.2%). The comorbidities like rheumatoid arthritis, hypothyroidism, and hyperlipidaemia were significantly higher in LYFE group (28.6%) than SOC group (11.4%), p=0.050 (Table 1).
| Demographic characteristics | LYFE (N=42) | SOC (N=44) | P-value* |
|---|---|---|---|
| Age (Years), Mean (SD) | 51.8 (12.0) | 56.5 (11.5) | 0.07 |
| Height (Cm), Mean (SD) | 164.2 (10.3) | 163.7 (9.3) | 0.81 |
| Weight (Kg), Mean (SD) | 72.9 (15.8) | 70.2 (12.6) | 0.39 |
| BMI (Kg/m2), Mean (SD) | 26.9 (4.7) | 26.2 (4.4) | 0.46 |
| Gender, n (%) | |||
| Male | 32 (76.2) | 32 (72.7) | 0.71 |
| Female | 10 (23.8) | 12 (27.3) | |
| Personal habits, n (%) | |||
| Alcohol | 11 (26.2) | 3 (6.8) | 0.02* |
| Tobacco smoking | 7 (16.7) | 5 (11.4) | 0.48 |
| Tobacco chewing | 2 (4.8) | 5 (11.4) | 0.24 |
| Clinical characteristic, n (%) | |||
| Diagnosis, n (%) | |||
| CAD | 24 (57.1) | 30 (68.2) | 0.29 |
| Post-ACS | 18 (42.9) | 14 (31.8) | |
| Previous PCI, n (%) | |||
| Yes | 14 (33.3) | 19 (43.18) | 0.77 |
| No | 28 (66.7) | 25 (56.81) | |
| Previous CABG, n (%) | |||
| Yes | 4 (9.5) | 5 (11.4) | 0.53 |
| No | 38 (90.5) | 39 (88.6) | |
| Indications for surgical intervention (Previous PCI snd CABG), n (%) | |||
| Asymptomatic | 0 (0) | 2 (13.3) | 0.11 |
| Unstable angina | 7 (38.9) | 5 (33.3) | |
| NSTEMI | 3 (16.7) | 5 (33.3) | |
| STEMI | 3 (16.7) | 3 (20.0) | |
| Others | 5 (27.8) | 0 (0) | |
| Comorbidities, n (%) | |||
| Hypertension | 32 (76.2) | 30 (68.2) | 0.41 |
| Diabetes | 17 (40.5) | 22 (50.0) | 0.38 |
| Both hypertension and diabetes | 13 (13.6) | 16 (36.4) | 0.6 |
| Others (Rheumatoid arthritis, CVA/TIA, Hypothyroidism and Hyperlipidaemia) | 12 (28.6) | 5 (11.4) | 0.05* |
| None | 0 (0) | 0 (0) | - |
Note: *Significance at P<05. ACS: Acute Coronary Syndrome; BMI: Body Mass Index; CAD; Coronary Artery Disease; CI; Confidence Interval; LYFE; Leading Your Future Experience; PCI; Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; NSTEMI: Non-ST-Elevation Myocardial Infarction; STEMI: ST-Segment Elevation Myocardial Infarction; SD: Standard Deviation; TIA: Transient Ischaemic Attack.
Table 1: Comparison of baseline characteristics in the LYFE group vs. SOC group.
Compliance and adherence evaluation
At 3 months, participants in the LYFE group (Figure 3A) showed higher medication adherence, with 85.4% (Never/Rarely: 43.9%, Once in a while: 41.5%) reporting 'hardly or no difficulty’ in remembering their medication compared to the SOC group (42.1%). In terms of physical exercise and diet, the LYFE group showed superior adherence with 92.7% and 95.1% of participants following their physical exercise (Figure 3B) and diet plans (Figure 3C), respectively, compared to 28.9% and 50% in the SOC group at 3-months. These results suggest that the LYFE intervention may be more effective in promoting adherence to treatment and lifestyle modifications compared to SOC.
Figure 3: Adherence to A) Medication; B) Physical exercise; C) Diet in LYFE and SOC groups across various follow-up periods in post-PCI patients with CAD and/or ACS. Note: ( ): LYFE; (
): LYFE; (  ): SOC.
): SOC.
Change in mean Dartmouth COOP Scale at 3 months
Upon considering the change in mean Dartmouth COOP Scale scores at 3 months, the LYFE group experienced significant improvements compared to the SOC group (p<0.001). Across various domains such as physical fitness (1.1 vs. 0.8), feelings (1.2 vs. 0.6), daily activities (1.0 vs. 0.5), social activities (1.2 vs. 0.5), pain (1.2 vs. 0.6), change in health (0.8 vs. 0.3), overall health (1.1 vs. 0.3), and quality of life (0.9 vs. 0.3), the LYFE group showed higher positive changes in mean scores compared to the SOC group (Table 2).
| Characteristics | 1st Month | 3rd Month | ||
|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | |
| Physical fitness | ||||
| Baseline, mean (SD) | 3.0 (0.5) | 3.4 (0.7) | 3.0 (0.5) | 3.4 (0.7) |
| Follow-up, mean (SD) | 2.8 (0.4) | 2.9 (0.4) | 1.9 (0.4) | 2.6 (0.5) |
| Mean difference (95% CI) | 0.2 (0, 0.4) | 0.5 (0.3, 0.7) | 1.1 (0.9, 1.3) | 0.8 (0.5, 1.1) |
| P-value* | 0.25 | <0.001 | ||
| Feelings | ||||
| Baseline, mean (SD) | 3.0 (0.6) | 3.2 (0.6) | 3.0 (0.6) | 3.2 (0.6) |
| Follow-up, mean (SD) | 2.8 (0.5) | 2.8 (0.4) | 1.8 (0.4) | 2.6 (0.6) |
| Mean difference, (95% CI) | 0.2 (0, 0.4) | 0.4 (0.2, 0.6) | 1.2 (1.0, 1.4) | 0.6 (0.3, 0.9) |
| P-value* | 0.05 | <0.001 | ||
| Daily activities | ||||
| Baseline, mean (SD) | 2.8 (0.7) | 3.1 (0.7) | 2.8 (0.7) | 3.1 (0.7) |
| Follow-up, mean (SD) | 2.8 (0.5) | 2.9 (0.4) | 1.8 (0.4) | 2.6 (0.6) |
| Mean difference, (95% CI) | 0 (-0.3, 0.3) | 0.2 (0, 0.4) | 1 (0.8, 1.2) | 0.5 (0.2, 0.8) |
| P-value* | 0.31 | <0.001 | ||
| Social activities | ||||
| Baseline, mean (SD) | 3.0 (0.5) | 3.1 (0.7) | 3.0 (0.5) | 3.1 (0.7) |
| Follow-up, mean (SD) | 3.0 (0.5) | 3.1 (0.7) | 1.8 (0.4) | 2.6 (0.6) |
| Mean difference, (95% CI) | 0.2 (0, 0.4) | 0.2 (0, 0.4) | 1.2 (1, 1.4) | 0.5 (0.2, 0.8) |
| P-value* | 0.31 | <0.001 | ||
| Pain | ||||
| Baseline, mean (SD) | 3.0 (0.5) | 3.2 (0.7) | 3.0 (0.5) | 3.2 (0.7) |
| Follow-up, mean (SD) | 2.8 (0.5) | 2.9 (0.4) | 1.8 (0.4) | 2.6 (0.7) |
| Mean difference, (95% CI) | 0.2 (0, 0.4) | 0.3 (0.1, 0.5) | 1.2 (1.0, 1.4) | 0.6 (0.3, 0.9) |
| P-value* | 0.31 | <0.001 | ||
| Change in health | ||||
| Baseline, mean (SD) | 2.7 (0.6) | 2.9 (0.7) | 2.7 (0.6) | 2.9 (0.7) |
| Follow-up, mean (SD) | 2.8 (0.5) | 2.9 (0.4) | 1.9 (0.3) | 2.6 (0.6) |
| Mean difference, (95% CI) | -0.1 (-0.3, 0.1) | 0 (-0.2, 0.2) | 0.8 (0.6, 1.0) | 0.3 (0, 0.6) |
| P-value* | 0.31 | <0.001 | ||
| Overall health | ||||
| Baseline, mean (SD) | 3.0 (0.5) | 3.0 (0.6) | 3.0 (0.5) | 3.0 (0.6) |
| Follow-up, mean (SD) | 2.8 (0.4) | 2.9 (0.4) | 1.9 (0.3) | 2.7 (0.5) |
| Mean difference (95% CI) | 0.2 (0, 0.4) | 0.1 (-0.1, 0.3) | 1.1 (0.9, 1.3) | 0.3 (0.1, 0.5) |
| P-value* | 0.25 | <0.001 | ||
| Social support | ||||
| Baseline, mean (SD) | 3.3 (0.6) | 3.1 (0.6) | 3.3 (0.6) | 3.1 (0.6) |
| Follow-up, mean (SD) | 3.0 (0.4) | 3.2 (0.5) | 4.2 (0.4) | 3.5 (0.6) |
| Mean difference (95% CI) | 0.3 (0.1, 0.5) | -0.1 (-0.3, 0.1) | -0.9 (-1.1, -0.7) | -0.4 (-0.7, 0.1) |
| P-value* | 0.04 | <0.001 | ||
| Quality of life | ||||
| Baseline, mean (SD) | 2.8 (0.4) | 3.0 (0.3) | 2.8 (0.4) | 3.0 (0.3) |
| Follow-up, mean (SD) | 2.6 (0.5) | 2.9 (0.5) | 1.9 (0.3) | 2.7 (0.5) |
| Mean difference (95% CI) | 0.2 (0, 0.4) | 0.1 (-0.1, 0.3) | 0.9 (0.7, 1.1) | 0.3 (0.1, 0.5) |
| P-value* | 0.01 | <0.001 | ||
Note: *Significance at P<.05. CI: Confidence Interval; LYFE: Leading Your Future Experience; SD: Standard Deviation; SOC: Standard of Care.
Table 2: Change in mean Dartmouth COOP Scale in the LYFE app. vs. standard of care.
The mean change in social support was slightly higher in the SOC group than the LYFE group (-0.4 vs. -0.9), this can be attributed to the fact that the LYFE app had no provisions for social support groups or forums.
This suggests that the LYFE intervention may have a significantly greater impact on the patient’s health-related quality of life over the 3-month period compared to SOC (Figure 4).
Figure 4: Mean difference in Dartmouth COOP scale from baseline to 3 months in LYFE and SOC group. Note: ( ): LYFE; (
): LYFE; ( ): SOC.
): SOC.
Comparing mean weight and BMI changes at 3-month
The mean change from baseline to 3 months in weight reduction were comparable between both the groups (LYFE: 0 vs. SOC: 1.5). Similarly, BMI also showed comparable mean changes (0 vs. 0.6) between both the groups. These differences were not statistically significant, suggesting that both interventions may have similar effects on weight and Body Mass Index (BMI) over 3 months (Table 3).
| Characteristics | 1st month | 3rd month | ||
|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | |
| Weight, (Kgs) | ||||
| Baseline, Mean (SD) | 73.0 (15.9) | 70.2 (12.6) | 73.0 (15.9) | 70.9 (12.9) |
| Follow-up, Mean (SD) | 72.1 (15.6) | 69.4 (12.3) | 73.1 (14.5) | 69.4 (13.3) |
| Mean change (95% CI) | 1 (0.6, 1.4) | 0.8 (0.5, 1.2) | 0 (-1.9, 1.9) | 1.5 (0.6, 2.4) |
| p-value* | 0.38 | 0.24 | ||
| Body Mass Index, BMI (Kg/m2) | ||||
| Baseline, Mean (SD) | 27.0 (4.8) | 26.2 (4.4) | 27.0 (4.8) | 25.6 (4.6) |
| Follow-up, Mean (SD) | 26.6 (4.7) | 25.9 (4.3) | 27.0 (4.7) | 25.6 (4.6) |
| Mean change (95% CI) | 0.4 (0.2, 0.5) | 0.3 (0.2, 0.4) | 0 (-0.7, 0.6) | 0.6 (0.3, 0.9) |
| p-value* | 0.48 | 0.19 | ||
Note: *Significance at P<.05. BMI: Body Mass Index; CI: Confidence Interval; LYFE: Leading Your Future Experience; SOC; Standard of Care.
Table 3: Comparing mean weight and BMI changes between LYFE vs. SOC groups.
Change in mean SBP, DBP, and PR at 3 months
The mean change from baseline to 3 months in SBP was higher in LYFE group when compared to SOC group (6.1 mmHg vs. 1.9 mmHg). The DBP levels were within range in both the groups, hence, no significant change was observed at follow-up. The mean change in pulse rate from baseline to the 3rd month was higher in the LYFE group compared to the SOC group (5.5 bpm vs. 0.1 bpm) (Table 4).
| Characteristics | 1st month | 3rd month | ||
|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | |
| Systolic Blood Pressure, SBP (mmHg) | ||||
| Baseline, mean (SD) | 125.0 (11.5) | 126.4 (13.5) | 125.0 (11.5) | 126.4 (13.5) |
| Follow-up, mean (SD) | 123.4 (17.7) | 127.6 (17.9) | 118.9 (13.6) | 124.5 (12.1) |
| Mean change (95% CI) | 1.6 (-4.9, 8.2) | -1.2 (-7.9, 5.5) | 6.1 (0.5, 11.6) | 1.9 (-4.0, 7.7) |
| P-value | 0.28 | 0.3 | ||
| Diastolic Blood Pressure, DBP (mmHg) | ||||
| Baseline, mean (SD) | 75.9 (9.9) | 77.4 (9.1) | 75.9 (9.9) | 77.4 (9.1) |
| Follow-up, mean (SD) | 79.1 (13.0) | 79.2 (10.2) | 78.4 (7.7) | 77.5 (7.7) |
| Mean change (95% CI) | -3.3 (-8.4, 1.8) | -1.8 (-5.9, 2.3) | -2.5 (-6.4, 1.4) | 0 (-3.9, 3.8) |
| P-value | 0.97 | 0.77 | ||
| Pulse rate, PR (bpm) | ||||
| Baseline, mean (SD) | 79.8 (8.4) | 78.7 (9.4) | 79.8 (8.4) | 78.7 (9.4) |
| Follow-up, mean (SD) | 78.7 (10.9) | 78.3 (13.9) | 74.3 (10.3) | 78.6 (11.0) |
| Mean change (95% CI) | 1.1 (-3.2, 5.4) | 0.4 (-4.6, 5.5) | 5.5 (1.4, 9.7) | 0.1 (-4.6, 4.8) |
| P-value | 0.99 | 0.08 | ||
Note: *Significance at P<.05. CI: Confidence Interval; LYFE: Leading Your Future Experience; SD: Standard Deviation; SOC: Standard Of Care.
Table 4: Change in mean SBP, DBP, and PR in the LYFE app. vs. SOC at 3 months.
The patients who achieved SBP<140 mmHg and DBP<90 mmHg were classified as having controlled BP range. In the LYFE group, 12.0% fell into the ‘uncontrolled’ category, whereas 21.0% in SOC group indicated that their BP did not meet the controlled range. Overall, the study revealed improved BP control in the LYFE group compared to SOC group participants (Figure 5).
Figure 5: Patients' BP status from baseline to 1-month and 3-month periods. Note: ( ): Control; (
): Control; (  ): Uncontrol.
): Uncontrol.
Comparison of biochemical assessment at 3-month intervention
It was observed that certain biochemical parameters like Low- Density Lipoprotein (LDL) (64.7 mg/dL vs. 64.0 mg/dL) and Haemoglobin (Hb) (13.1 mg/dL vs. 13.1 mg/dL) remained stable in SOC group, whereas slight reduction was observed in LDL (66.5 mg/dL vs. 61.3 mg/dL) and Hb levels (13.6 mg/dL vs. 13.3 mg/dL) in LYFE group. Similarly, higher trends in mean differences were observed in LYFE group for parameters like HDL (2.2 mg/dL vs. 1.8 mg/dL) and fasting blood sugars (1.6 mg/dL vs. -13.1 mg/dL) compared to SOC groups. It is noteworthy that HbA1c increased from 7.3% to 7.5% in SOC group, whereas LYFE was successful in achieving reduced levels of HbA1c (7.6% vs. 6.9%). Hence, it can be inferred that LYFE appears to show favourable trends, such as LDL reduction and improved HbA1c levels, attributed to improved adherence to medications and app assistance (Table 5).
| Characteristics | 1st Month | 3rd Month | ||
|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | |
| Low Density Lipoprotein, LDL (mg/dL) | ||||
| Baseline, mean (SD) | 66.1 (46.2) | 65.8 (26.0) | 66.5 (43.6) | 64.7 (23.3) |
| Follow-up, Mean (SD) | 59.1 (30.4) | 63.1 (23.6) | 61.3 (25.9) | 64.0 (26.5) |
| Mean difference (95% CI) | 7.1 (-18.6, 32.8) | 2.8 (-18.3, 23.8) | 5.2 (-15.2, 25.6) | 0.7 (-18.0, 19.3) |
| P-value* | 0.7 | 0.75 | ||
| High Density Lipoprotein, HDL (mg/dL) | ||||
| Baseline, mean (SD) | 38.2 (11.7) | 42.5 (14.0) | 38.1 (11.3) | 40.6 (13.0) |
| Follow-up, mean (SD) | 34.6 (8.6) | 42.1 (14.7) | 35.9 (7.5) | 38.8 (11.0) |
| Mean difference (95% CI) | 3.6 (-3.2, 10.3) | 0.4 (-11.7, 12.5) | 2.2 (-3.2, 7.7) | 1.8 (-7.2, 10.8) |
| P-value* | 0.09 | 0.33 | ||
| Triglycerides, TG (mg/dL) | ||||
| Baseline, mean (SD) | 143.3 (56.3) | 157.7 (84.4) | 142.1 (49.6) | 156.6 (79.2) |
| Follow-up, mean (SD) | 128.5 (50.4) | 141.8 (62.3) | 154.8 (63.5) | 148.6 (61.7) |
| Mean difference (95% CI) | 14.7 (-20.4, 49.9) | 16 (-46.8, 78.7) | -12.6 (-45.0, 19.8) | 8 (-45.1, 61.1) |
| P-value* | 0.52 | 0.77 | ||
| Total Cholesterol, TC (mg/dL) | ||||
| Baseline, mean (SD) | 134.1 (48.3) | 133.8 (46.4) | 142.4 (29.9) | 138.7 (28.0) |
| Follow-up, mean (SD) | 119.4 (35.1) | 128.7 (25.8) | 133.4 (34.9) | 132.5 (27.9) |
| Mean difference (95% CI) | 14.7 (-13.2, 42.7) | 9 (-18.6, 36.5) | 5 (-16.3, 26.3) | 6.1 (-14.8, 27.1) |
| P-value* | 0.28 | 0.67 | ||
| Haemoglobin, Hb (mg/dL) | ||||
| Baseline, Mean (SD) | 14.0 (2.1) | 13.0 (1.24) | 13.6 (2.03) | 13.1 (1.2) |
| Follow-up, Mean (SD) | 13.6 (1.8) | 13.2 (1.24) | 13.3 (1.42) | 13.1 (1.1) |
| Mean difference (95% CI) | 0.4 (-1.0, 1.8) | -0.1 (-1.2, 0.9) | 0.3 (-0.7, 1.3) | 0.1 (-0.8, 0.9) |
| P-value* | 0.49 | 0.58 | ||
| Fasting Blood Glucose, FBS (mg/dL) | ||||
| Baseline, mean (SD) | 125.2 (67.9) | 106.5 (22.9) | 116.6 (61.0) | 110.8 (21.9) |
| Follow-up, mean (SD) | 119.2 (42.0) | 117.1 (74.0) | 114.9 (55.8) | 123.9 (52.1) |
| Mean difference (95% CI) | 6.0 (-32.2, 44.3) | -10.6 (-59.3, 38.1) | 1.6 (-32.3, 35.6) | -13.1 (-44.2, 17.9) |
| P-value* | 0.92 | 0.63 | ||
| Postprandial Blood Glucose, PBS (mg/dL) | ||||
| Baseline, mean (SD) | 220.0 (135.8) | 142.7 (0) | 162.3 (54.2) | 142.72 (0) |
| Follow-up, mean (SD) | 167.2 (100.7) | 193.0 (0) | 165.55 (98.1) | 216.2 (0) |
| Mean difference (95% CI) | NA | NA | NA | NA |
| P-value* | - | - | ||
| Glycated haemoglobin, HbA1c (%) | ||||
| Baseline, mean (SD) | 7.7 (2.6) | 6.9 (1.6) | 7.6 (2.4) | 7.3 (1.9) |
| Follow-up, mean (SD) | 7.0 (2.1) | 6.9 (1.5) | 6.9 (1.8) | 7.5 (2.0) |
| Mean difference (95% CI) | 0.7 (-0.9, 2.3) | 0 (-1.2, 1.3) | 0.7 (-0.6, 2.0) | -0.1 (-1.6, 1.3) |
| P-value* | 0.29 | 0.63 | ||
| Creatinine, (mg/dL) | ||||
| Baseline, mean (SD) | 1.1 (0.3) | 1.02 (0.3) | 1.0 (0.3) | 1.0 (0.2) |
| Follow-up, mean (SD) | 1.0 (0.2) | 1.1 (0.3) | 1.0 (0.2) | 1.0 (0.2) |
| Mean difference from baseline (95% CI) | 0.1 (-0.1, 0.3) | 0 (-0.3, 0.2) | 0.1 (-0.1, 0.2) | 0 (-0.2, 0.2) |
| P-value* | 0.84 | 0.36 | ||
Note: *Significance at P<.05. CI: Confidence Interval; LYFE: Leading Your Future Experience; NA: Not Available due to the insufficient data; SD: Standard Deviation; SOC: Standard of Care.
Table 5: Comparison of biochemical assessment in LYFE vs. SOC group at 3-month.
Comparison of clinical outcomes at 3 months
Upon assessing the clinical outcomes like cardiovascular deaths, the LYFE group reported one (2.4%) death at 1-month follow-up, none were reported in SOC group. However, at 3 months, SOC group reported two (5.3%) cardiovascular deaths, none were reported in LYFE group (Table 6).
| Characteristics | 1st month/30 days | 3rd month/90 days | ||
|---|---|---|---|---|
| LYFE (n=42) | SOC (n=44) | LYFE (n=42) | SOC (n=44) | |
| Clinical outcomes (mortality, myocardial infarction, stroke, target vessel revascularization, heart failure admission and emergency visit), n (%) | 1.0 (2.4%) | 0 (0) | 0 (0) | 2.0 (5.3%) |
| P-value* | 0.29 | 0.14 | ||
Note: *Significance at P<.05. LYFE; Leading Your Future Experience; SOC; Standard of Care; NA: Not Available due to insufficient data.
Table 6: Comparison of clinical outcomes in LYFE vs. standard-of-care patients.
Hospitalization history
There were no hospitalizations for either group at 1-month. One patient (2.4%) in LYFE group had a planned 1-day hospitalization at 3-months, whereas 2 (5.3%) patients in SOC group had unplanned hospitalizations lasting an average of 3.5 days at 3-months (Table 7).
| Characteristics | 1st month/30 days | 3rd month/90 days | ||
|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | |
| Number of Hospitalization, n (%) | 0 (0) | 0 (0) | 1.0 (2.4)# | 2.0 (5.3)## |
| Mean number of days, n (%) | 0 (0) | 0 (0) | 3 day | 3.5 days |
| P-value* | NA | 0.51 | ||
Note: *Significance at P<.05. LYFE: Leading Your Future Experience; SOC: Standard of Care; N: -Not Available due to insufficient data; #1-Critical SVD (planned); ## Hypoglycemia (unplanned) and Left ventricular failure with AKI (unplanned).
Coronary Artery Disease (CAD) is a multifactorial disease. The modifiable risk factors for CAD includes elevated blood pressure, high cholesterol levels, diabetes, obesity, sedentary lifestyle and unhealthy diet [14]. The American College of Cardiology/American Heart Association (ACC/AHA) and the European Society of Cardiology (ESC) recommend intensive risk factor management combined with enhanced adherence to a healthy lifestyle, proper exercise, and cardioprotective therapies in patients with ACS [5,15]. Secondary prevention involves modifying risk factors to reduce mortality, decrease the frequency of cardiac events, and improve quality of life. This can be made possible with individualized treatment plans, thorough vital monitoring, patient and caregiver education, and by providing emergency services. This paves the path for the collaboration of DTx with the conventional SOC in patients with CAD and ACS.
The comprehensive support provided by the LYFE app, including personalized coaching and educational modules, can facilitate positive lifestyle modifications, which are essential for managing CAD and ACS. This was evident from the present study where higher medication adherence was seen in LYFE group than SOC group (85.4% vs. 42.1%). This improved adherence was consistent in medications, exercises (92.7% vs. 28.9%), and diet plans (95.1% vs. 50.0%). This was similar to the observations recorded in a pilot study by Li et al., which demonstrated that an app-based DTx in combination with conventional care resulted in significant improvement in medication adherence at 12 months [16]. Furthermore, it has also been observed that the benefits offered by DTx are at peak when used with conventional pharmacological therapies [17].
Arterial hypertension is a significant risk factor in cardiovascular disease. This is apparent from the fact that the majority of patients with MI have a history of hypertension. This is consistent with the present study where 76.2% of the patients in LYFE as well as 68.2% of patients in SOC group reported history of hypertension. It has been observed that initiating proper blood pressure control prior to discharge ensures enhanced prognosis and secondary prevention in CAD patients [18]. As evidenced in this study, LYFE DTx can render this tangible with its accurate monitoring of BP and alerts for off-range readings. The mean change from baseline to 3 months in SBP was higher in LYFE group when compared to SOC group (6.1 vs. 1.9), suggesting that the LYFE intervention may have resulted in a greater reduction in SBP compared to the SOC. Multiple studies based on DTx facilitated self-monitoring app observing patients for 3 and 12 months have demonstrated significant reduction in SBP [10,16,19,20]. This study also observed slight reductions in LDL levels (66.5 mg/dL vs. 61.3 mg/dL) and HbA1c levels (7.6% vs. 6.9%) from baseline to 3 months in LYFE group. This was in resemblance to the observations of a meta-analysis by Groot et al., where a significant reduction was seen in HbA1c levels (-0.561 to -0.410) in patients with telemedicine interventions [21].
Quality of Life (QoL) plays a prominent role in crafting personalized management plans for patients with chronic diseases [22]. A study by Murphy, et al., observed that post-PCI patients face activity constraints, emotional fluctuations, and social withdrawal [23,24]. This drawback sets the groundwork for the LYFE DTx, as it emerges with improved Dartmouth COOP Scale scores at 3 months in this study, where the LYFE group experienced greater improvements compared to the SOC group, across various domains such as physical fitness (1.1 vs. 0.8), feelings (1.2 vs. 0.6), daily activities (1.0 vs. 0.5), social activities (1.2 vs. 0.5), pain (1.2 vs. 0.6), change in health (0.8 vs. 0.3), overall health (1.1 vs. 0.3), and quality of life (0.9 vs. 0.3). These improved readings can be attributed to the personalized diet, fitness plans, dedicated LYFE care managers, and psychological counsellors. This was similar to the findings in CONENCT intervention, where 31% of patients recorded enhanced medication adherence, 40% reported taking measures to improve the mental well-being, 47% reported increased physical activity and 61% reported healthier eating patterns with eHealth strategies [25,26].
Another challenge faced by cardiologists is the higher frequency of readmissions post-PCI. A multicentre study by Biswas, et al., observed that 1 in 10 patients suffers an unplanned hospital readmission within 30 days post-PCI. This could be mitigated by personalized treatment and discharge procedures, along with thorough outpatient follow-up modules for patients [27]. Digital therapeutics like LYFE can help combat the increased rate of rehospitalizations post-PCI, as evidenced by the results observed in this study, where only one patient (2.4%) in the LYFE group had a planned readmission, whereas 2 (5.3%) patients in the SOC group had unplanned hospitalizations lasting an average of 3.5 days at 3 months. This highlighting the potential of LYFE to reduce healthcare utilization and improve patient outcomes.
The synergistic effect of conventional SOC, improved adherence to medications, diet and exercise, regulation of vital signs, controlled blood pressure, improved blood sugar levels, and enhanced quality of life offered by LYFE DTx in combination with SOC can change the course of cardiac care especially in patients with CAD and/or ACS. These findings consistent with the prior studies emphasizes that DTx is a practical and effective approach to managing CAD and/or PCI. However, additional research is necessary to safely establish the clinical significance of these findings and comprehensively explore the impact of DTx on the management of CAD.
Future directions
The DTx products demands higher involvement from patients than any other element of the healthcare facilities, hence thorough consideration of the socio-economic background of the patients is necessary to achieve the intended therapeutic outcomes [28]. Furthermore, considerations for scalability, cost-effectiveness, and patient preferences should be explored to facilitate the integration of digital therapeutics into routine clinical practice.
The present study highlights the potential of integrating DTx into SOC for patients with CAD and/or ACS, post-PCI). It was observed that LYFE app intervention, combined with SOC, significantly improved medication adherence, physical exercise adherence, and diet habits compared to SOC alone. In addition to this, the LYFE intervention led to notable enhancements in QoL. Furthermore, the LYFE intervention was associated with favourable trends in clinical outcomes, including reductions in SBP and PR, improved blood pressure control, and positive changes in biochemical parameters such as LDL cholesterol and HbA1c levels. LYFE intervention was also linked to a lower frequency of hospital readmissions, highlighting its potential to reduce healthcare utilization and improve patient outcomes.
To conclude, this study contributes to the growing body of evidence supporting the integration of DTx with SOC into cardiovascular care to improve patient outcomes and healthcare delivery.
This was a single centered study, which may limit the findings to other settings or populations. Variability in patient demographics, healthcare practices, and resource availability across different centres could affect the results. Longitudinal studies with extended follow-up periods would provide more insights into the durability of the observed improvements.
This study employed a prospective design, allowing for the collection of real-time data and minimizing recall bias, which enhances the reliability and validity of the study findings. It also assessed a wide range of clinical, behavioural, and biochemical outcomes, including medication adherence, physical exercise, diet habits, quality of life, hospital admissions, Major Adverse Cardiovascular Events (MACE), and biochemical parameters, which could provide a thorough evaluation of the intervention's efficacy and safety. By incorporating digital therapeutics into standard care, the study offers a practical solution to enhance patient outcomes and improve healthcare delivery in this population.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors would like to thank the study investigators, staff, and all participants who took part in the study. Medical writing support was provided by Dr. Madhura Donde (Alpha MD Pvt Ltd), Mumbai.
I confirm that the research study was approved by the Institutional Ethics Committee (ECR/233/Indt/GJ/2015/RR-21).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Potdar A, Gharat C, Patel K (2024) Comparing Effects of Smartphone Application Based Standard of Care vs. Standard of Care in Acute Coronary Syndrome and Coronary Artery Disease Patients: A Real-World Study. J Clin Exp Cardiolog.15:884.
Received: 18-Apr-2024, Manuscript No. JCEC-24-30857 ; Editor assigned: 22-Apr-2024, Pre QC No. JCEC-24-30857 (PQ); Reviewed: 06-May-2024, QC No. JCEC-24-30857 ; Revised: 13-May-2024, Manuscript No. JCEC-24-30857 (R) ; Published: 20-May-2024 , DOI: 10.35248/2155-9880.24.15.884
Copyright: ©2024 Potdar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.