Gynecology & Obstetrics
Open Access
ISSN: 2161-0932
ISSN: 2161-0932
Research Article - (2017) Volume 7, Issue 1
Aims and 0bjectives: To determine and evaluate the difference/s, in terms of tumor control and side effects, between weekly and three weekly cisplatin concomitant with radical course of radiotherapy for locally advanced carcinoma of cervix.
Materials and methods: The study was conducted on sixty previously untreated, histopathologically proven patients of locally advanced carcinoma of uterine cervix. The patients were treated with External Beam Radiotherapy (EBRT) 50Gy in 25 fractions over 5 weeks and concomitant cisplatin, followed by intra-cavity HDR brachytherapy (ICBT) 700cGy to point A, once in a week for three weeks. The patients were assigned randomly either of two groups of 30 patients each. In Group I (Study Group) the patients received three weekly cisplatin 75 mg/m2 for 2 cycles while in Group II (Control Group) the patients received weekly cisplatin 40 mg/m2 for 5 cycles. Evaluation of response and toxicity was done weekly during treatment and monthly thereafter. The data thus obtained was assessed and analysed using SPSS version 20.0 statistical tool.
Results: There was statistically insignificant higher incidence of hematological , skin , mucosal toxicity and GI toxicity in group I. Stage wise disease status at the end of sixth month follow up was as follows: Stage IIA-NED* (80% vs. 100%), RD** (20% vs. 0%);Stage IIB - NED (80% vs. 76.67%), RD (20% vs. 23.53%); Stage IIIA - NED (60% vs. 100%), RD (40% vs. 0%); Stage IIIB- NED (60% vs. 60%), RD (40% vs. 40%). Tumor response was not significantly different in the two groups with respect to age distribution, rural/ urban distribution, histopathological distribution and treatment interruption (*No Evidence of Disease **Residual Disease). Conclusion: Three weekly cisplatin, concomitant with radiation seems to be the potential, effective and acceptable alternate as standard of treatment for locally advanced carcinoma cervix; especially for increased work load and limited resource facilities.
Keywords: Weekly versus three weekly cisplatin; Concomitant; Chemoradiation; Carcinoma cervix
Globally, carcinoma cervix is the 6th most common cancer and 8th most common cause of death [1]. Current estimates indicate that every year 122844 women are diagnosed with cervical cancer and 67477 die from the disease in India [2]. In countries like India, the disease most often diagnosed in advanced stage when the disease has already spread to adjacent structures including vagina, paracervical and paravaginal soft tissues, urinary bladder and rectum and by this time pelvic lymph nodes are also involved in 20-25% cases [3,4]. A combined modality approach is necessary for the management of patients with cervical cancer [5]. For locoregionally advanced disease (stage IIB, III, IVA) radiation therapy is the primary treatment modality with or without chemotherapy [1]. The high local failure rate in patients with locally advanced carcinoma of the cervix treated with standard radiation has spurred interest in radiosensitization [1,3]. Concomitant chemoradiation is one of the several avenues being explored in this regard. In 1999 National Cancer Institute (NCI) issued a clinical alert, chemoradiotherapy became the standard of care in treating women with cervical cancer when it showed that chemoradiation led to 6% improvement in 5-year survival [6-8]. In recent years, various chemotherapeutic agents have been tried for concomitant chemoradiation in carcinoma cervix, either as single agent or in combination to increase the effect and decrease the local failure rates like vinorelbine, irinotecan, gemcitabine, 5-fluorouracil etc, but investigators are yet to find optimal dose and dosing schedule for them. In present study, it has been tried to compare the compliance to and toxicity of weekly cisplatin 40 mg/m2 and three weekly cisplatin 75 mg/ m2 concurrent with radiotherapy [5]. A possible hypothesis of this study is that the higher peak concentration of cisplatin may be more critical in enhancing synergy of chemoradiation than the weekly cisplatin exposure [8,9]. The high peak concentration of cisplatin may be more effective not only in enhancing the synergy of chemoradiation but also in eliminating micrometastases, with resulting decrease of local failure and distant metastasis and improvement of survival eventually with a tolerable toxicity profile [10,11].
The study was conducted between 2013 and 2015 on sixty previously untreated, histopathologically proven patients of carcinoma of cervix, attending the Department of Radiotherapy and Department of Obstetrics and Gynaecology, Pt. B.D. Sharma PGIMS Rohtak for definitive treatment by radiation therapy. The patients with Karnofsky Performance Status more than or equal to 70 and normal hematological, hepatic and renal functions were included in the study.
The patients were divided randomly in two groups of 30 patients each by draw of lots.
Both the groups were treated with Concomitant chemoradiation with 50Gy/25F/5 wks to whole pelvis along with cisplatin followed by Intracavitary HDR brachytherapy 7 Gy to point A three times, once in a week. The overall duration of treatment was 8 weeks (56 days).
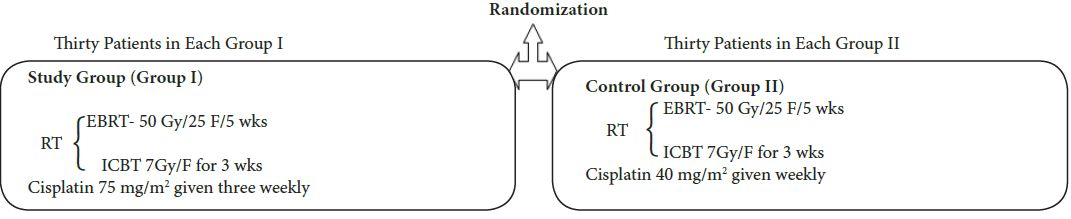
1. EBRT was delivered by Cobalt - 60 teletherapy and brachytherapy by HDR micrselectron machines. Assessment of toxicities was done weekly during treatment in both the groups as per WHO toxicity criteria
2. RTOG Acute Radiation Morbidity Scoring Criteria. Tumor response was assessed as per WHO response criteria. All the patients were followed up fortnightly for first month and then, monthly up to 6 months.
3. Weekly and three weekly cisplatin with dose of 40 mg/m2 and 75 mg/m2 under pre and post hydration of 1 L of intravenous fluids.
4. Premedications given include: Dexamethasone 16 mg IV stat, Pheniramine 25 mg IV stat, Ondansetron 8 mg IV stat, Ranitidine 50 mg IV stat (all this medications were administered 30 minutes before the initiation of chemotherapy.
5. Potassium chloride IV, Magnesium sulphate IV, 100% Mannitol IV administered during the chemotherapy.
Dose escalation or reduction was not done during the course of treatment in both the groups.
Patients with treatment interruption more than 1 week were excluded from the study.
For data analysis, SPSS version 20.0 was used. Statistical significance was considered when p value was less than 0.05.
Observations were made at the end of treatment and last follow up. Response for the purpose was determined by clinical examination. Radiological examination, fine needle aspiration cytology or a biopsy was carried out in clinically suspicious cases. Patient characteristics are shown in (Tables 1-4).
| Characteristics | Group I (n=30) | Group II (n=30) | P value | |
|---|---|---|---|---|
| Mean Age | 55.4 ± 11.22 | 47.96 ± 11.00 | 0.182 | |
| Symptoms at presentation | Abnormal Discharge P/V* | 14(46.67) | 17(56.67) | 0.161 |
| Abnormal Bleeding P/V* | 21(70) | 22(73.33) | ||
| Post coital bleeding | 0 | 3(10) | ||
| Abdominal pain | 5(16.67) | 2(6.67) | ||
| Back pain | 5(16.67) | 6(20) | ||
| Constipation | 0 | 0 | ||
| Difficulty in micturition | 2(6.67) | 0 | ||
| Menstrual history | Premenopausal | 4(13.33) | 10(33.33) | 0.067 |
| Postmenopausal | 26(86.67) | 20(66.67) | ||
| Histopathology | MDSCC** | 29(96.67) | 28(93.33) | 0.554 |
| PDSCC*** | 1(3.33) | 2(6.67) | ||
| Morphology | Ulceroproliferative | 26(86.67) | 29(96.67) | 0.161 |
| Infiltrative | 4(13.33) | 1(3.33) | ||
| Stage | IIA | 10(33.33) | 6(20) | 0.251 |
| IIB | 10(33.33) | 17(56.67) | ||
| IIIA | 5(16.67) | 2(6.67) | ||
| IIIB | 5(16.67) | 5(16.66) | ||
| Treatment interruption | Present | 10 (33.33) | 8 (26.67) | 0.573 |
| *P/V Per Vaginum Histopathological distribution (**Moderately differentiated squamous cell carcinoma ***Poorly differentiated squamous cell carcinoma) |
||||
Table 1: Patient characteristics.
| Treatment characteristics | Group I | Group II |
|---|---|---|
| Radiotherapy dose | 50 Gy | 50 Gy |
| Planned number of fractions | 25 fractions | 25 fractions |
| Dose per fraction | 200cGy | 200cGy |
Table 2: Treatment characteristics.
| Cisplatin dose (75mg/m2 IV)* | Group I n**(%) |
|---|---|
| 110 mg | 4(13.34) |
| 120 mg | 21(70) |
| 130 mg | 1(3.33) |
| 135 mg | 1(3.33) |
| 140 mg | 2(6.67) |
| 150 mg | 1(3.33) |
| *- based on the BSA (body surface area) **- no of patients |
|
Table 3: Cisplatin dose (75 mg/m2 IV).
| Cisplatin dose (40mg/m2 IV)* | Group IIn**(%) |
|---|---|
| 50 mg | 6(20) |
| 60 mg | 20(66.67) |
| 70 mg | 2(6.66) |
| 75 mg | 2(6.67) |
| *- dose calculated as per surface area of each patient. **- no of patients. |
|
Table 4: Cisplatin dose (40 mg/m2 IV).
Tumor response was not significantly different in the two groups with respect to age distribution, rural/ urban distribution, histopathological distribution and treatment interruption (Tables 5-7 and Figure 1).
| Parameters assessed | Highest grade of toxicity | Group I (no. of patients) |
Group II (no. of patients) |
p value | |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | WHO | Grade 2 | 7(23.33) | 6(20) | NS* |
| TLC (1000/ mm3) | WHO | Grade 2 | 2(6.67) | 3(10) | NS* |
| Platelet(lacs /mm3) | WHO | Grade 2 | 0 (0) | 1 (1) | NS* |
| SGOT (U/L) | WHO | Grade 2 | 0 (7) | 0 (26) | NS* |
| SGPT (U/L) | WHO | Grade 2 | 0 (17) | 0 (29) | NS* |
| S.ALP (U/L) | WHO | Grade 2 | 0 (0) | 0 (0) | NS* |
| Nausea/vomiting | WHO | Grade 2 | 4(13.33) | 4(13.33) | NS* |
| Blood urea (mg/ dL) | WHO | Grade 2 | 0 (9) | 1 (6) | NS* |
| Serum creatinine (mg/ dL) | WHO | Grade 2 | 0 (6) | 0 (15) | NS* |
| Acute skin reactions | RTOG | Grade 3 | 4(13.33) | 1(3.33) | NS |
| Acute mucosal reactions | RTOG | Grade 3 | 7(23.33) | 5(16.67) | NS |
| Acute lower gi toxicity | RTOG | Grade 2 | 8(26.67) | 9(30) | NS |
| Weight loss at the end of the treatment | SWOG | Grade 2 | 9(30) | 13(43.33) | NS |
| *: Nothing significant | |||||
Table 5: Table demonstrating toxicity observed during treatment.
| Group 1(No. of patients) | Group 2 (No. of patients) | P Value | |
|---|---|---|---|
| Treatment interruption <1 week | 8 | 7 | NS |
| Treatment interruption >1 week | 2 | 1 | NS |
| Treatment interruption due to chemotherapy related toxicities | 7 | 6 | NS |
| Treatment interruption due to radiation related toxicities | 3 | 2 | NS |
Table 6: Table demonstrating treatment interruption and reasons.
| Parameters Assessed | Group I | Group II | p value |
|---|---|---|---|
| Skin | 4 | 4 | Ns |
| Subcutaneous | 5 | 6 | Ns |
| Mucosal | 5 | 5 | Ns |
| Bladder | 7 | 6 | Ns |
| Rectal | 8 | 6 | Ns |
Table 7: Table demonstrating late radiation induced toxicities at last follow up (median duration 9 months).
Recent meta-analysis reported that chemoradiotherapy leads to 6% improvement in 5 year survival when compared with radiotherapy alone. The concomitant chemoradiation represents a major treatment option in patients with bulky stage IB-IIA disease, IIB-IVA disease and resectable stage IB-IIA disease with poor prognostic factors. The rationale for this is that concurrent chemotherapy inhibits the repair of sublethal damage from radiation, synchronises cells to a particular radiosensitive phase of cell cycle and is cytotoxic in vitro. As a potent sensitizer of cancer cells, currently, cisplatin has been the "traditional partner" of external beam irradiation for locally advanced carcinoma of the cervix based on the results of some randomized trials. These trials have shown that the use of concurrent chemoradiation results in a 30% to 50% decrease in the risk of death as compared to radiotherapy alone [8,9].
In this study, we tried to compare the compliance to and toxicity of weekly cisplatin 40 mg/m2 and three weekly cisplatin 75 mg/m2 administration concurrent with radiotherapy. This study was planned with the view is that the higher peak concentration of cisplatin may be more critical in enhancing synergy of chemoradiation than the weekly cisplatin exposure. Although the dose-response slope of cisplatin is not steep, the response of tumor to cisplatin has been shown to increase as the peak concentration of cisplatin increases up to 100 mg/m2 [9,10].
The high peak concentration of cisplatin may be more effective not only in enhancing the synergy of chemoradiation but also in eliminating micrometastases, with resulting decrease of local failure and distant metastasis and improvement of survival eventually with a tolerable toxicity profile [9,10].
Similar studies conducted earlier Ryu et al. on 104 patients with histologically proven Stage IIB-IVA cervical cancer concluded that there was no statistically significant difference in compliance between the two arms. Grade 3-4 neutropenia was more frequent in the weekly arm than in the three weekly arm. The overall 5-year survival rate was significantly higher in the three weekly arm than in the weekly arm [10]. Another trial conducted by Nagy et al. on 326 patients with locally advanced squamous cell cervical carcinoma concluded that the 5-year survival rate obtained through the 2 RCT regimens are not statistically different [11].
In present study tumor response was not significantly different in the two groups with respect to age distribution, rural/urban distribution, histopathological distribution and treatment interruption. The most important determinant of the tumor response was stage, as no other factor was found to influence response rate. The difference in hematological toxicity and gastrointestinal toxicity) observed in the two groups at the end of treatment was found to be statistically insignificant. The worst acute skin and mucosal reactions observed during treatment and by the end of treatment in was mainly grade I. Grade II and grade III was slightly more in the study group but the difference was statistically insignificant. Treatment interruption for more than a week due to acute radiation toxicities was more common in the study group. There were insignificantly more cutaneous, Subcutaneous, Mucosal and lower GI toxicities in the study group observed as late radiation toxicity.
It may be concluded from this study that there is no statistically significant difference in tumor control, radiation induced skin and mucosal toxicity, hematological, upper gastrointestinal and lower gastrointestinal toxicities when concomitant chemo-radiation with cisplatin is given either three weekly or weekly.
The limitations of the study include small sample size and short follow up duration. Taking into account the comparable response rates and comparable treatment related toxicities; the study is suggestive that the future clinical trials should consider to investigate the potential effectiveness of three weekly cisplatin along with radiation in the treatment of locally advanced cervix cancer in view of increased work load and limited trained medical, paramedical staff and limited medical facilities in developing countries with low resources like India.