
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 3
Background: Monoclonal Immunoglobulins (MIs) are screened using Serum Protein Electrophoresis (SPE) and Capillary Zone Electrophoresis (CZE). In SPE, proteins are separated in an agarose gel by their electrophoretic mobility and charge distribution. MIs are present in the gamma globulin region, but can also be found in the beta or alpha-2 region, which can make quantitation and interpretation inaccurate. Elevated beta globulin may be an indication of iron deficiency anemia, 3rd trimester pregnancy or oral contraceptive use. CZE resolves the betaglobulin as two peaks; beta-1 (transferrin) and beta-2 (complement proteins (C3)) regions. We present six cases that illustrate the resolving power of 6-band CZE vs. 5-band SPE to detect IgA and IgM MI bands hidden within the beta region.
Methods: SPE and CZE were performed using the Helena SPIFE 3000 and sebia capillarys 2 systems respectively. Confirmation of MIs was by Immunofixation Electrophoresis (IFE).
Results: For all patients, SPE reported a wide range in electrophoretic pattern and MI detection. CZE was able to identify M-proteins not quantitated on SPE. We observe a pattern whereby the concentration difference between beta-1 and beta-2 become reversed (“flipped”) in the presence of a hidden IgA MI by SPE.
Conclusion: Six-band CZE is superior to 5-band SPE due to its ability to separate the beta region into beta-1 and beta-2, thus allowing better detection of hidden MIs. Further studies with a larger sample size may be warranted to establish a rule for IFE reflexing when the beta-2 fraction is greater than the beta-1 by the CZE method.
Monoclonal gammopathy; Monoclonal immunoglobulins; Serum protein electrophoresis; Capillary zone electrophoresis; Immunofixation electrophoresis
MI: Monoclonal Immunoglobulins; SPE: Serum Protein Electrophoresis; CZE: Capillary Zone Electrophoresis; IFE: Immunofixation Electrophoresis; MG: Monoclonal Gammopathy; MGUS: Monoclonal Gammopathy of Undetermined Significance; M-protein: Monoclonal-protein; MM: Multiple Myeloma; AGE: Agarose Gel Electrophoresis; ISE: Immunosubtraction Electrophoresis
Monoclonal Gammopathy (MG) is demonstrated by identification of Monoclonal Immunoglobulins (MI) or free light chains in serum and/or urine. Characterization requires protein separation by SPE or CZE and immunotyping.
We present cases that evaluate the resolving power of 5-band SPE and IFE to detect IgA and IgM MI comigrating within the beta region [1]. We report 6-band CZE to be superior to 5-band SPE due to its ability to separate the beta region into beta-1 and beta-2.
Also, the CZE can improve on follow-up monitoring of patients when M-spike levels are used as an indicator of tumor burden and resistance to therapy.
The detection of occult IgA monoclonal immunoglobulins is critical for diagnosing and monitoring multiple myeloma and other plasma cell dyscrasias. Traditional methods such as Immunofixation Electrophoresis (IFE) and Serum Protein Electrophoresis (SPEP) have been widely used for this purpose. However, advancements in electrophoretic techniques have introduced alternative systems that promise improved sensitivity, specificity, and overall diagnostic accuracy. This comparative study aims to evaluate various electrophoretic systems employed in detecting occult IgA monoclonal immunoglobulins, emphasizing their technical capabilities, diagnostic performance, and clinical utility.
Electrophoresis, the fundamental principle underlying these systems, separates proteins based on their size, charge, and other physicochemical properties. SPEP and IFE have been the cornerstone of monoclonal protein detection, leveraging differences in protein migration patterns to identify abnormal immunoglobulins. SPEP provides an initial screening, while IFE offers more detailed characterization of immunoglobulin isotypes. Despite their widespread use, these methods have limitations in detecting low-abundance monoclonal proteins, particularly IgA, due to their lower serum concentrations and tendency to form polymeric structures.
Recent innovations have led to the development of advanced electrophoretic systems such as Capillary Electrophoresis (CE) and mass spectrometry-based techniques. CE, for instance, offers higher resolution and faster analysis times compared to traditional gel-based methods. It also requires smaller sample volumes, enhancing its applicability in clinical settings. Mass spectrometry, on the other hand, provides unparalleled sensitivity and specificity, enabling the detection of monoclonal proteins at very low concentrations. These advanced systems promise to overcome the limitations of conventional methods, particularly in the early detection of occult IgA monoclonal immunoglobulins, which is crucial for timely therapeutic intervention.
This comparison seeks to elucidate the strengths and weaknesses of each electrophoretic system, providing a comprehensive overview that aids clinicians and laboratory professionals in selecting the most appropriate diagnostic tools for detecting IgA monoclonal immunoglobulins. By doing so, it aims to enhance diagnostic accuracy, improve patient outcomes, and pave the way for the integration of cutting-edge technologies in clinical diagnostics.
The diagnosis of Monoclonal Gammopathies (MGs) is demonstrated by identification of monoclonal immunoglobulins in serum and/or urine. In MGs, there is overproduction of an Immunoglobulin (Ig) from clonal plasma cells (monoclonal (M)- protein). MG includes less malignant phenotypes (ALamyloidosis, smoldering multiple myeloma and Monoclonal Gammopathy of Undetermined Significance (MGUS)) and malignant phenotypes (Multiple Myeloma (MM), Waldenstrom’s macroglobulinemia and lymphoplasmacytic lymphoma) [2].
MG is present in 3%-5% of patients above 50 years and progression to multiple myeloma from less malignant forms occurs at about 1% annum. Less malignant forms are asymptomatic despite characteristic plasma cell clone or Monoclonal Proteins (M-Proteins) or Immunoglobulins (MI). It is therefore important for sensitive technologies to detect Mproteins [3].
The primary method of identification and characterization of Mproteins has been Agarose Gel Electrophoresis (AGE). Serum Protein Electrophoresis (SPE), an AGE method, separates proteins based on their electrophoretic mobility and charge distribution (electroendosmosis) to produce a pattern of major fractions: Albumin, alpha-1, alpha-2, beta and gamma globulins (Figure 1). In a normal serum, the gamma fraction has a broad distribution due to thousands of secreted immunoglobulin clones by normal plasma cells hence termed polyclonal gamma globulins [4]. With the recent development of high resolution SPEs (6-band SPEs) there is the ability to separate the gamma fraction into two peaks; gamma-1 and gamma-2. However, just about 14% of all laboratories use the 6-band SPE (50% of all SPE labs) per 2018 ELP-B proficiency testing challenges.
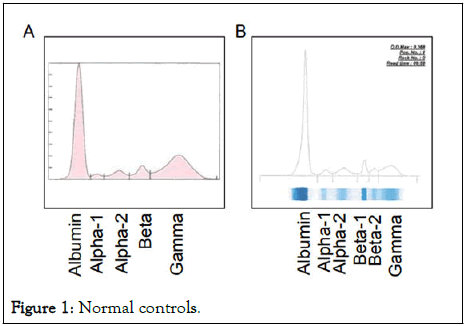
Figure 1: Normal controls.
Capillary Zone Electrophoresis (CZE), an alternative to SPE, is a rapid, cost effective and time saving method that allows the separation of charged molecules in a buffer-filled negatively charged column under an applied voltage (30 kv). The method is partially automated with assay completion in about 1 minute. CZE has a higher separation efficiency (increased sensitivity) and specificity compared to 5-band SPE in the identification of Mproteins. Its electrophoretic pattern is similar to SPE with the separation of the beta globulin region into beta-1 and beta-2 peaks (Figure 1).
Identification of an M-protein or homogenous band on an SPE or CZE requires immunotyping by Immunofixation Electrophoresis (IFE) or capillary zone Immunosubtraction Electrophoresis (ISE). However, a negative SPE or CZE result does not exclude a MG. IFE has improved sensitivity (LOD~100 mg/L) over SPE (LOD~500 mg/L). Typically, IFE is performed by immunoprecipitating with antisera to the heavy chains IgG, IgA, IgM and the light chains kappa and lambda following electrophoresis [5]. Visualization of a free light chain without an associated heavy chain is reflexed to an additional IFE gel with antisera for IgD and IgE.
Despite the importance of these assays in MG diagnosis, they have technical and interpretational challenges. We present six cases that illustrate the resolving power of 5-band SPE and 6-band CZE to detect the presence of an IgA or IgM MIs comigrating with the beta peak [6].
SPE and CZE were performed using the Helena SPIFE 3000 and Sebia capillarys 2 systems respectively. Protein fractions were quantified by densitometry (Helena) with total serum protein concentration (determined on the Beckman DxC) as the reference or direct UV absorbance (Sebia) at 200 nm. Total protein and immunoglobulin types were quantified by Beckman DxC [7]. Confirmation of MIs was by Immunofixation Electrophoresis (IFE) (Figure 2).
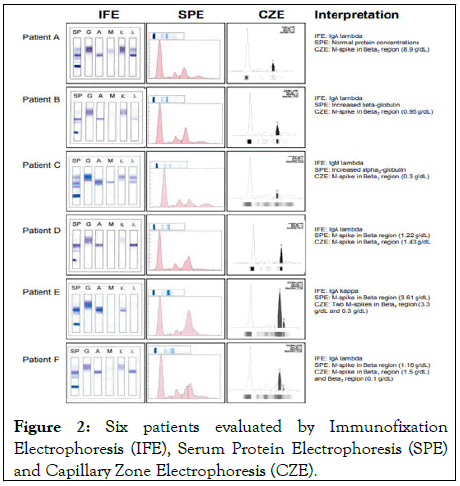
Figure 2: Six patients evaluated by Immunofixation Electrophoresis (IFE), Serum Protein Electrophoresis (SPE) and Capillary Zone Electrophoresis (CZE).
We evaluated 6 patients on which IFE and SPE were ordered and compared their SPE results to CZE. For SPE, a spectrum of globulin concentration and M-protein detection was observed across patients: A normal electrophoretic pattern and protein concentration (patient A), increased beta (patient B) and alpha 2 globulin (patient C) and monoclonal peaks adjacent to the beta globulin (patient D-F). In comparison with CZE, all patients had identifiable monoclonal bands (M-spike) in the alpha 2 region (patient C), beta 1 (patient C and F) and beta 2 regions (patient A, B, D and E) that were quantifiable and corroborated IFE results.
In addition, CZE was able to identify additional monoclonal bands (Patients E and F) not identified by SPE. For all patients with a hidden IgA MI, we observed a reversed (“flipped”) concentration difference between beta-1 and beta-2 on CZE. In all patients except patient C, turbidimetric immunoglobulin quantification on Beckman DxC showed increase in the IgA (Table 1) that was confirmed by IFE. An IgM lambda monoclonal protein was observed for patient C (Table 1) [8-9].
| DxC | Reference range | Patient A | Patient B | Patient C | Patient D | Patient E | Patient F |
|---|---|---|---|---|---|---|---|
| Immunoglobulin | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL |
| IgG | 750-1560 | 784 | 659 | 957 | 326 | 261 | 535 |
| IgA | 80-450 | 677 H | 913 H | 118 | 1100 H | 4376 H | 1978 H |
| IgM | 46-304 | 52 | 25 | 196 | 25 | 25 | 44 |
Table 1: Immunoglobulin quantification by an immuno-turbidometric method on Beckman-DxC.
Compared to 5-band SPE, CZE showed greater sensitivity for detecting occult IgA and IgM monoclonal proteins in the beta and alpha regions respectively. Internationally about 68% of laboratories use SPE and 31.4% use CZE in the screening and diagnosis of MGs. Currently, SPE makes up about 59.2% of the 2018 ELP-B proficiency testing challenges [10]. Physicians screening for MG usually request SPE or CZE with reflex to IFE or immunosubtraction electrophoreses. Occult immunoglobulins create a challenge when initial screening for MG is SPE alone or prior to reflexing.
Patients with disease may be missed (patient A) or interpreted as having acute infection (patient C). Elevated beta globulin (patient B), of which the major fraction is transferrin, may be an indication for iron deficiency anemia, 3rd trimester pregnancy or the use of oral contraceptives. Our observation of an IgM monoclonal protein migrating in the alpha-2 region (patient C) on SPE is uncommon and suggest that in addition to IgA, other occult monoclonal proteins, including IgG (an observation not shown), can migrate anodally in the beta and alpha regions and be missed [11].
These abnormalities may be missed on 5-band SPEs in part due to transferrin or C3 obscuring visualization of M-proteins in the beta fraction. The ability of 6-band CZE to separate beta-1 and beta-2 peaks enhances the detection of M-protein in this area. Thus high beta globulins (and rarely alpha 2 globulins) on 5-band SPEs should trigger IFE testing.
Our cases also suggest that follow-up monitoring using SPE can be challenging. SPE can fail to properly quantify M-spikes whose levels are used as an indicator of tumor burden and resistance to therapy (patient D-F) [12]. Its limitation to identify biclonal bands (patient E and F) due to overlap can cause misinterpretation of a resistant clone as possible relapse in subsequent testing [13].
While our observations are made on 6 patients, we corroborate previous reports. Litwin, et al reported that agarose gels were able to detect 11/17 (79%) of IgA monoclonal spikes detected by CZE, indicating that this can be a common occurrence of IgA monoclonal proteins. In fact, high resolution SPEs (capable of beta splitting) and CZE has shown improved capabilities for MI identification and is recommended for patient evaluation. We further report that IgM and IgG monoclonal proteins migrating further anodally than usually observed can be hidden on 5-band SPEs. Thus, compared to 5-band SPE, 6-band CZE provides improved capabilities for the detection of occult MIs in the beta region. However, there is perfect agreement with 5-band SPE in samples that do not contain monoclonal proteins.
There are no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Muluhngwi P, Yin T, Cost KM, Elin RJ (2024) Comparison of Electrophoretic Systems to Detect Occult IgA Monoclonal Immunoglobulins. J Clin Chem Lab Med. 7:295.
Received: 24-Mar-2020, Manuscript No. JCCLM-24-3765; Editor assigned: 30-Mar-2020, Pre QC No. JCCLM-24-3765 (PQ); Reviewed: 13-Apr-2020, QC No. JCCLM-24-3765; Revised: 01-Jun-2024, Manuscript No. JCCLM-24-3765 (R); Published: 28-Sep-2024 , DOI: 10.35248/2736-6588.24.7.295
Copyright: © 2024 Muluhngwi P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.