Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2017) Volume 10, Issue 7
In the present research work, the physicochemical studies of surface active Cyclohexanoxy carbonyl Pyridinium single tailed and Gemini amphiphiles had been compared. Thermal stability, size of aggregates in aqueous system and interaction with a globular protein, Bovine Serum Albumin (BSA), was done by using TGA analysis, DLS, UV-visible, steady-state fluorescence and synchronous fluorescence and TEM respectively. On the basis of the experiments, it had been concluded that the Gemini amphiphile possessed less cytotoxicity, high thermally stability, form small sized compact aggregates and stable complex with BSA. The results have further been supported by higher value of Stern-Volmer quenching constant (KSV) and a higher binding constants for Gemini amphiphiles as compared to single tailed amphiphiles. Moreover, on the basis of synchronous fluorescence spectra and UV spectra, it has been concluded that both the amphiphiles mainly interact with tryptophan residues. The less cytotoxicity values and stabilization of secondary structure of BSA in low concentration of ionic liquids implies that these ionic liquids can replaced the conventional surfactants in the detergent industries and used as a potential vehicles for the drug and gene delivery.
Keywords: Gemini amphiphiles; Single tailed amphiphiles; Bovine serum albumin; cytotoxicity
Bioactive peptides
A compound which can be used as a solvent must be eco-friendly and easily biodegradable. This type of stipulations can be fulfilled only when the degradable reactants used for the synthesis of ionic liquids or introduction of degradable functionality like amide or ester in to the moiety of the resulted product [1,2] or by synthesizing the ionic liquids with high surface activity and low Critical Micelle Concentrations (CMC) [3-5]. Ionic liquids (ILs) have replaced traditional organic solvent, because of low vapor pressure [6,7], high thermal stability, nonflammability and capability to dissolve wide range of compounds [8,9]. Single tailed amphiphiles are the compounds with one hydrophobic tail linked with one hydrophilic head group. Gemini amphiphiles are the class of compound with twin hydrophobic tails linked with hydrophilic head with or without spacer [10]. Gemini amphiphiles are more beneficial as compared to single tailed ionic amphiphiles, because of their high surface activity, low critical micelle concentration [11], better solubilizing ability and greater ability to form stable structures with globular proteins [12,13]. In the recent instance, the interaction of globular protein with single tailed and Gemini amphiphiles had been investigated by the different research groups [14-17]. The binding of amphiphiles with proteins usually depends upon the moiety and other features of amphiphiles. BSA serves as a depository protein for a large range of compounds. It is one of the largely studied proteins because of its structure homology with the Human Serum Albumin (HSA) [18,19].
In the present research work, we have used ester based Cyclohexanoxy carbonyl Pyridinium single tailed and gemini amphiphiles, synthesized in our lab [20,21] and compared their thermal stability, size of aggregates, cytotoxicity and interaction with globular protein bovine serum albumin. To study the interactions of amphiphiles with BSA, we have used different techniques like ultra-violet spectroscopy, fluorescence spectroscopy, Dynamic Light Scattering (DLS) and Transmission Electron Microscopy (TEM). UV and fluorescence spectroscopic studies have been employed because the change in the wavelength of emission maximum (λmax) and intensity parameters found to be sensitive to protein conformation and can be used to investigate the interactions between BSA and ionic liquids. DLS and TEM studies are used to see the change in conformation of the amphiphiles in the presence of BSA.
Materials
Gemini Amphiphiles (GA) and Single Tailed amphiphiles (ST) (Figure 1) were synthesized in our lab by an earlier reported procedure [18,19]. Bovine Serum Albumin (BSA) (MW=66 kDa) was purchased from Central drug house (New Delhi, India) and was used as received. 0.01 M buffer, pH 7.2, was made from 0.01 M KH2PO4 and 0.01 M sodium hydroxide. Potassium dihydrogen phosphate and sodium hydroxide were purchased from Merck, India. Millipore water was used in all experiments.
Thermal gravimetric analysis
Thermal stability of both the amphiphiles had been investigated by Perkin Elmer Pyris 1 Thermal Gravimetric Analyzer (TGA). Both the samples were completely dried by rotatory vaccum pump. Both the samples were added in to the aluminum pans under the inert atmosphere at a heating rate of 10°C/min.
The size of the studied cationic amphiphiles has been measured by the Zetasizer Nano ZS (Malvern Instrument Ltd.) UK. The inbuilt peltier device controls the temperature with an accuracy of ±0.1 K. The 1.0 mM aqueous solution of both amphiphiles has been filtered through 0.45 μM pore sized filter before measurement.
Cytotoxicity
The cytotoxicity of, 1.0 mM aqueous solution, both synthesized amphiphiles was carried out on C6 glioma (cancerous brain cell line, passage number 65) by using MTT assay to calculate the IC50 value of both the synthesized ionic liquids. Cells were seeded at a density of 10 × 103 cells/ml in 96 well microtiter plates. After 24 hours of seeding, cells were treated with the test compounds over a concentration range from 10 to 900 μM. After 24 hrs of treatment, the cells were incubated at temperature 37°C with MTT containing medium (10 mg/ 10 ml of serum free medium). The resulting blue formazan crystals formed by viable cells were solubilized by 100 μl of Di-methyl sulfoxide (DMSO) and maximum absorbance was taken at 595 nm using a Multiskan PLUS plate reader (Thermo Scientific, USA).
Ultraviolet spectra
The ultraviolet spectra of BSA and BSA in ionic liquid were recorded on a UV-1601 PC Shimadzu ultraviolet-visible spectrophotometer. The concentration of aqueous solution of BSA was 1.5 × 10-6 mol L-1 and of an amphiphiles was 25 mM in all experiments. The experiment was carried out at 298.15 K, λ=190-400 nm, and with path length 1.0 cm.
Fluorescence spectra
The fluorescence measurements of BSA in the absence and presence of BSA was carried out on LS 55 fluorescence spectrometer, Perkin Elmer, equipped with a Peltier system (PTP 1) at 25.0 ± 0.1°C. The excitation wavelength 280 nm and path lengths 1.0 cm was used to examine the emission spectra of protein.
Synchronous fluorescence spectra
Synchronous fluorescence spectra were also recorded by the same spectrofluorometer. The difference between excitation wavelength and emission wavelength was fixed (Δλ=λem-λex). The characteristics information about the Tyrosine (Tyr) or Tryptophan (Trp) residues was obtained, when Δλ was at 20 or 60 nm. The excitation and emission slits were set at 10/2.5 nm for all the experiments.
Transmission scanning microscopy was carried out by JEM-2100, Jeol, Japan, working at an accelerating voltage of 120 kV, to study the morphology of ionic liquids with or without BSA. A 0.45 μm aperture size millipore membrane filter was used for the filtration of prepared samples. All the samples were filtered prior to experiment. A 5 μl of sample was loaded on to the collodion-cotted copper grids already placed on the filter paper and allowed to remain the system as such for air-dry.
Thermal Gravimetric Analysis (TGA)
Thermal stability of the Gemini and single tailed amphiphiles were compared (Figure 1) by determining the start temperature (Tstart) and onset temperature (Tonset). The (Tstart) temperature is explained as point where the degradation of the compound starts and (Tonset) temperature is defined as the intersection point of the baseline weight from the start of the experiment and tangent of weight loss vs temperature curve. From the experimental statistics, it has been cleared that the Gemini amphiphiles possess reasonably good thermal stability as compare to their single tailed amphiphiles. Thermal stability of the compound has been affected by the number of factors like functional group present in the hydrophobic tail [22], heterocyclic moiety [23], symmetry of the cation [3] and size of anions [24]. Our experimental results bare that the Gemini analogue was more thermally stable as compared to single tailed amphiphiles (Table 1), due to the symmetry factors. Figures 2a-2d shows the percent mass and the rate of mass loss as a function of the temperature. The thermal degradation of the gemini amphiphile was observed as a large weight loss (76.2%) at 259.3°C due to loss of (C19H24N2O2)2+ and two bromide ion, whereas in case of single tailed analogues a large weight loss (71.9%) at 223.5°C due to loss of (C8H10NO)+ and bromide ion.
| Amphiphiles | Tstart (°C) | Tonset (°C) |
|---|---|---|
| GA | 200.4 | 247.4 |
| ST17 | 177.9 | 212.9 |
Table 1: Start and onset temperature of gemini and single tailed amphiphiles.
Dynamic light scattering
Dynamic Light Scattering (DLS) was performed to determine the size distribution of aggregates of single tailed and Gemini amphiphiles beyond CMC. DLS results (Figure 3) cleared that the range of hydrodynamic radius (RH) for Gemini amphiphiles is 0.60 nm and for single tailed amphiphiles is 1.09 nm. On comparing the aggregates size of Gemini and single tailed amphiphiles, the aggregates size of Gemini amphiphiles is less than single tailed amphiphiles. The observed results may be due to the difference in the counterion binding (β) [18,19]. The counterion binding for the Gemini amphiphiles was 0.74 and for single tailed amphiphiles was 0.52, which resulted in to the formation of compact micellar aggregates.
Cytotoxicity
In the present research work, C6 glioma cells were treated with varying concentrations of the ionic liquids (10-900 μM) and cytotoxicity was evaluated by performing MTT assay. In case of Gemini amphiphiles significant increase in cell viability was observed in cells treated with 10 μM and 50 μM of test compound and significant decrease in percent of viable cells was observed at concentrations higher than 350 μM. In case of single tailed amphiphile significant decrease in cell viability was observed in cells treated with 250 μM and higher concentrations of test compounds. The experimental results clearly demonstrate that the ester functionalized ionic liquids were found to be very less toxic. Cytotoxicity of the surface active ionic liquids (SAILs) was expressed as percentage of cell viability in terms of IC50 value i.e., the concentration of the testing compound (in μM) that causes the death of the 50% of the living cells. The experimental results had been shown in Figures 4a and 4b, which disclosed that the investigated Cyclohexyloxy SAILs are not cytotoxic up to a concentration of 350 μM. The introduction of ester functionality and Cyclohexyloxy in to the hydrophobic tail makes the compound less toxic. Furthermore, the present compounds have also found to be less toxic as compared to earlier synthesized Imidazolium and Pyridinium amphiphiles [3,4].
Ultraviolet spectra
The ultraviolet spectra found to be important technique to look into the change in conformation of protein molecule during interactions [25]. Figures 5a and 5b show the ultraviolet spectrum of BSA aqueous solution (1.5 × 10-6 M) in curve 1. The main absorption peaks of the BSA have been observed in the range of 200 to 400 nm. The excitation of BSA in the ultraviolet region is due to the different aromatic amino acids like histidine, tyrosine (Tyr), tryptophan (Trp) and phenylalanine (Phe). The absorption peak around 280 nm was assigned to the tryptophan (Trp), tyrosine (Tyr) and phenylalanine (Phe) due to the n-π* transition of aromatic region and absorption peak around 220 nm was assigned to the carbonyl (C=O) containing amino acids like aspartate (Asp), Asparagines (Asn), glutamate (Glu) and glutamine (Gln) residues due to π-π* transition of the protein peptide backbone [26].
The results indicated the change in ultraviolet absorption spectra on addition of cationic amphiphiles. The change in intensity and wavelength of BSA on addition of cationic amphiphiles are indicative of change in the microenvironment of the Trp and Tyr residues on addition of cationic amphiphiles. These changes also explain the deformation of peptide bond. The close and ordered peptide chain changes in to the loose and disordered chain [27].
The change in absorbance on addition of cationic amphiphiles also indicated that the cationic head groups of the amphiphiles bind to amino acid residues via H-bond. In case of single tailed amphiphiles, a gradual blue shift of maximum wavelength confirmed the interaction and unfolding of BSA, which transferred the BSA towards more hydrophobic region (Figures 5c and 5d). Change in maximum wavelength (λmax) has also been observed in case of Gemini amphiphiles, but at lesser extent, which show that the mechanism of binding of Gemini amphiphiles with BSA is different than the single tailed amphiphiles. The value of concentration at which complete unfolding of BSA occurred for Gemini amphiphiles is 0.165 mM and for single tailed it is 0.415 mM.
Fluorescence spectra
The polarity of the microenvironment in BSA solution was found to be weaker, due to the strong hydrophobicities of Trp, Tyr and Phe residues [28]. Fluorescence quenching can be explained as a bimolecular process in which fluorescence quantum yield decreases, without undergoing any change in fluorescent emission spectra. In the present case, the effect of cationic gemini and single tailed amphiphiles on the intrinsic fluorescence of BSA was examined by the fluorescence quenching.
Fluorescence quenching
The fluorescence spectroscopy was performed to give information about the changes in the peptide bond conformation of BSA protein induced by the interaction with surface active gemini and single tailed amphiphiles. Figure 6a shows the variation in the emission intensity of BSA on addition of cationic amphiphiles with excitation wavelength of 280 nm. On addition of cationic amphiphiles, it can be seen that the quantum yield of fluorophores decreases. For the BSA-amphiphiles system, the addition of cationic amphiphiles decreased the intensity of BSA fluorescence. It was connected with the formation of BSAamphiphiles complex and unfolding of the BSA structure. In the case of single chain amphiphiles (Figure 6b), a gradual blue shift of the emission maxima was observed with increasing amphiphiles concentration.
Thus the maximum of emission wavelength shifting towards shorter wavelength in case of single tailed amphiphiles bared that BSA may have transferred to a more hydrophobic environment. There is also decrease in fluorescence intensity due to the hydrophobic environment in both the cases. The shift of fluorophore towards a more hydrophobic environment leads to the unfolding of proteins. Figure 6c shows the variation of maximum emission wavelength with respect to concentration of amphiphiles (GA) and (ST). The values of concentration at which the BSA get saturated for the Gemini Amphiphiles (GA) is 0.141 mM, while for single tailed amphiphiles it was found 0.462 mM. After these concentration values, the maximum emission intensity becomes almost constant. These results are in consonance with the Uv-visible spectroscopic results. These values are taken as Critical Aggregation Concentration (CAC). In this case the electrostatic interactions also occur between the cation and anion of amphiphiles and BSA. Fluorescence quenching is mainly of two types dynamic and static. Dynamic quenching is due to the collisions between the fluorophore (BSA) and the quencher (amphiphiles) or static quenching arising from the formation of a ground state complex between the fluorophore and the quencher. The contact between the fluorophore and the quencher molecules was necessary in both the cases for the fluorescence quenching [29].
Stern-volmer equation 1 was used to estimate the quenching in terms of quenching constant.
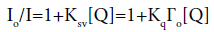 (1)
(1)
In the above equation, Io and I stand for the fluorescence intensities in the absence and presence of the amphiphiles, respectively [Q] is the concentration of the amphiphiles and Ksv=Kq Гo is the Stern-Volmer quenching constant. Kq is the bimolecular quenching constant, and Гo is the lifetime of the fluorophore in the absence of the quencher. Thus, the slope of the Stern- Volmer plot of Io/I versus [Q] can be used as quenching constant.
Figure 7a showing the linear relationship was observed for Io/I versus Q for cationic amphiphiles when excited at λex=280 nm. The bimolecular quenching constant Kq for GA and ST system was calculated from Ksv=KqГo (Гo=10-8 s) (Table 2). These quenching constants were found to be higher than the largest possible bimolecular quenching constant 1010 L mol-1s-1 in aqueous medium, which suggested that the fluorescence quenching was not initiated by dynamic collision, which proved the specific interaction between BSA and cationic amphiphiles. The bimolecular quenching constant Kq value was also find to be more for gemini cationic amphiphiles as compared to single tailed analogues.
| Amphiphiles | Ksv × 103 (L mol-1) | R | Kq× 108 (L/mols) | K × 103 (L mol-1) | n | R |
|---|---|---|---|---|---|---|
| GA | 18.74 | 0.9861 | 18.74 | 8.5680 | 3.23 | 0.9939 |
| ST | 0.202 | 0.9975 | 0.202 | 0.0018 | 1.06 | 0.9981 |
Table 2: Stern-volmer quenching constant for the cationic amphiphiles-BSA system.
Binding parameters of BSA with cationic amphiphiles
When small molecules bind independently to a set of equivalent sites on a macromolecule, the equilibrium between the free and the bound molecules can be determined according to the method described by Ref. [30].
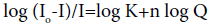 (2)
(2)
Where Io and I are the fluorescence intensities before and after the addition of the amphiphiles, K is the binding constant and n is the average number of binding sites per BSA. The intercept and the slope of double logarithm curves from, (Equation 2; Figures 7a and 7b) can be used to determine K and n. The data in the Table 2 shows the high affinity binding constant for GA, which indicated a relatively strong interaction between GA and BSA compared to single tailed amphiphile.
Synchronous fluorescence
Synchronous fluorescence screening is a useful technique to acquire information about the molecular environment in the vicinity of the chromophore molecules. The environment of the amino acid residues is evaluated by measuring the shift in the maximum emission wavelength corresponding to the changes of the polarity around the chromophore molecules. As referring to proteins assays-Tryptophan, Tyrosine and Phenylalanine are the three main intrinsic fluorophores of the BSA protein. Phenylalanine is not much sensitive as compare to the Trp and Tyr, therefore the quantum yield of this residue is rather low as compare to other residues [28], therefore the emission from this residue can be ignored. The synchronous fluorescence spectra with Δλ=20 and 60 nm are characteristics of Tyr and Trp residues, respectively [31]. To explore the structural change of BSA, induced by the addition of ionic liquids, synchronous fluorescence spectra of BSA with various amounts of ionic liquid are recorded with Δλ=20 and 60 nm. The intrinsic fluorescence of BSA is almost completely contributed by Trp alone because the fluorescence intensity of BSA for Δλ=60 nm is higher than that for Δλ=20 nm. Tryptophan emission observed at shorter wavelength in a hydrophobic environment. The results indicated that the presence of cationic amphiphiles lead to fluorescence quenching of BSA, while the characteristics emission wavelength kept unchanged in case of gemini amphiphiles, but it shows slight shift in case of single chain amphiphiles. It can also be seen that much more quenching was recorded when Δλ of 60 nm (Figures 8a and 8b) was adopted. However, single tailed amphiphiles, the fluorescence intensity for Δλ=60 nm decreased while it increased for Δλ=20 nm. These results suggested that the Trp residue is much closer to the cationic part of the amphiphiles as compare to Tyr residue in the interaction system (Figures 8a and 8b).
Transmission Electron Microscopy (TEM)
The adsorption of globular protein BSA on the micellar surface has also been visualized by transmission electron microscopy (TEM). The TEM images of compound GA and ST has been examined in the absence (Figure 9a and 9b) and presence (Figure 9c and 9d) of BSA. In case of single tailed amphiphile images shows the spherical micelles in the absence of BSA. However, in the presence of BSA, the formation of micro size cell like vesicles has been observed. The spherical micelles have also been observed in case of Gemini amphiphiles, (GA). But in the presence of BSA, it changed in to fibril shaped small aggregates. This shows that the binding interactions of single tailed has been different from the Gemini amphiphiles. Figure 9d shows that in the binding of BSA with single tailed amphiphiles, BSA resides on the surface of spherical micelles, which causes the unfolding of the BSA proteins.
In the present research, the gemini and corresponding single tailed amphiphiles have been compared on the basis of their thermal stability and aggregation size. From the experimental results, it had been concluded that the Gemini amphiphiles exhibited more thermal stability due to its symmetry. The synthesized compounds have also been found to be less cytotoxic; therefore can be easily used in drug delivery, gene delivery and other biochemical applications.
The DLS studies revealed that the Gemini amphiphiles formed compact micelles as compared to single tailed amphiphiles. The binding interactions of these amphiphiles with globular protein BSA had also been studied by UV, steady state fluorescence and synchronous fluorescence. The Gemini amphiphiles has possessed more interaction with BSA, as explained on the basis of higher value of Ksv, as compared to single amphiphiles. The synchronous spectra and UV spectra of amphiphiles also show that the unfolding of proteins is mainly due to the interaction between amphiphiles and Trp residues of BSA [32]. TEM studies revealed that the mechanism of binding of single chain amphiphiles is different from that of Gemini amphiphiles. The binding of single chain amphiphiles leads only to the unfolding of BSA. However, the binding of Gemini amphiphiles plays two opposite roles in the unfolding and the stability of the BSA structure. At low Gemini amphiphiles/BSA ratio, it can stabilize the secondary structure of BSA although the tertiary structure of BSA is lost. This may be due to the two hydrophobic tails in case of Gemini amphiphiles, which allows the hydrophobic linkages between the non-polar residues of BSA. According to the TEM images, single tailed amphiphiles formed large size vesicles on interaction with BSA, while Gemini amphiphiles formed small sized compact fibrillar structure on interaction with BSA. Our experimental results proved that the Gemini ionic liquids are more surface active and possessed more thermal stability and binding ability with BSA and therefore can be preferred over the single tailed amphiphiles at the industrial level.
Our results also inspired us to design more functionalized ionic liquids with better surface active properties and moderate thermal stabilities, which ccan be easily replaced conventional surfactants in detergent industries used for the stabilization of proteins.
The author Rajni Aggarwal is thankful for the financial support by the UGC BSR in the form of research fellowship and grant.