Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2023)Volume 14, Issue 3
Cosmos is an aromatic plant with Essential Oils (EOs) used in food, flavouring, and medicine. A comparative investigation of the chemical composition and antimicrobial activities of the leaf EOs of Cosmos bipinnatus Cav. (cultivated) and Bidens ferulifolia (Jacq.) (wild) from Bangladesh has been conducted, whereas the composition of wild EO is being studied for the first time. Both EOs were extracted using the hydro distillation method, and the chemical composition of both EOs was identified by Gas Chromatography–Mass Spectrometry (GC-MS). The antimicrobial activity of cultivated EO was tested against ten pathogenic bacteria and six pathogenic fungi using the disc diffusion method. Even at 2 g disc-1, cultivated EO was able to inhibit all of the test microorganisms from growing. The value of EO was evaluated for the minimum inhibitory concentration (MIC, 100-300 ppm) and minimum bactericidal concentration (MBC, 200-400 ppm). At 100 ppm, all of the test fungi had 100% of their radial mycelial growth stopped by essential oils. The Minimum Fungicidal Concentration (MFC) was between 50 and 300 ppm, and the Minimum Inhibitory Concentration (MIC) was between 200 and 400 ppm. The wild-type EO contained (Z)-β-ocimene (28.76%), germacrene D (19.19%), 2,6-dimethyl-1,3,5, -octatetraene, E.E (17.12%), caryophyllene (5.40%) and elixene (4.64%), whereas the cultivated type confirmed (Z)-β-ocimene (48.29%), trans-3-caren-2-ol (19.21%), sabinene (9.36%), germacrene D (6.10%) and β-pinene (4.18%). The main constituent (Z)-β-ocimene has a 40% higher content in the cultivated type than the ‘wild’ type. The cultivated type of Cosmos EO has promising antimicrobial activities.
Pseudomonas aeruginosa; Fermentation; Steam distillation; Insecticidal; Antioxidant
The medicinal plants "Cosmos" (a genus mainly grown in South Africa, China, East Asia, India, Australia, and Europe) include Cosmos bipinnatus Cav. (cultivated) and Bidens ferulifolia (Jacq.) (wild), both of which are members of the Asteraceae family [1]. Both are medium-sized flowering, herbaceous, and half-hardy annual medicinal plants ranging from 0.6 to 1.8 m in height with an open and sprawling habit. Among these, the genus Cosmos comprises 26-42 types that have spread throughout Europe, Asia, and Africa [2]. The Cosmos has a lot of potential for Essential Oils (EOs) [3]. The EO isolated from Cosmos contained monoterpenes (69.62%) and sesquiterpenes (22.73%), which demonstrated notable inhibitory effects against both Gram-negative and Gram-positive bacteria [4]. The researcher employed EOs instead of synthetic antioxidants since they are recognized as natural antioxidants and are non-toxic [5]. Due to EOs' larvicidal, analgesic, anti-inflammatory, antioxidant, antifungal, and anticancer activities, as well as the fact that many EOs exhibit antimicrobial activities [6]. However, due to their high reactivity, low water solubility, high volatility, limited stability, potent organoleptic properties, and hydrophilic nature, they are not frequently employed in food products [7,8]. Additionally, compared to EOs, some natural materials, such as wax and honey, may modify their nutritional characteristics or have lower levels of polyphenols [9]. The presence of more than 250 bioactive components in EO also gives it exceptional natural antibacterial and antioxidant qualities, which are beneficial for food preservation [7]. These EOs can therefore be exploited as flavouring ingredients in the food industry, as medicines and cosmetics, as well as insecticidal, antioxidant, anti-inflammatory, anti-allergic, and anticancer agents [10,11]. Many bioactive constituents have been isolated from Cosmos caudatus leaf and root extracts, which have been shown to have anti-diabetic, antihypertensive, antimicrobial, anti-osteoporotic, and anti-inflammatory activities in various clinical, in vitro, and in vivo studies [12-17]. Terpine-4-ol is indicated as the principal antimicrobial agent, which was isolated from C. bipinnatus Cav. [18,19]. The chemical profile and stereo chemical categories of EOs vary in terms of the number of molecules present, and most of the EOs are “Generally Recognized as Safe” (GRAS) found in the extracts of plant parts [20,21]. In 2019, 2′-hydroxy-4,4′-dimethoxychalcone, butein, α-hydroxybutein, luteolin, tricin, quercetin-5-O-β-D-glucopyranoside, quercetin, chysoeriol, balanophonin B, β-sitosterol, E-ferulic acid hexacosyl ester, 3,4-dihydroxybenzoic acid, n-butyl gallate, and benzoic acid was reported for the first time from the genus Cosmos [22]. According to previous study, the major components of C. bipinnatus Cav. are β-elemene (15-17%), β-caryophyllene (15-17%), germacrene D (10-21%) and bicyclogermacrene (12-15%) [23]. In an another earlier investigation, it was discovered that the Cosmos included significant amounts of (E)-β - Ocimene (50.23%), germacrene D (13.99%), sabinene (9.35%), α-cadinol (4.27%), α-farnesene (3.15%) and terpinene-4-ol (3.04%) [4]. However, the differences in the chemical constituents of this plant may be due to the different geographical locations. Hence, there is a relationship between Cosmos's antimicrobial activity and its EO composition. The EO isolated from natural sources may significantly contribute to disclosing nutraceutical and functional food components by overcoming the low stability and efficacy difficulties through modification. Plant chemical compositions must be identified in order to assess the effectiveness of their bioactive constituents in antibacterial activities because EOs has the potential to be medicinal uses. In addition, the development of new antibacterial and antifungal agents, which are natural products and recognized as significant sources of biologically active substances, including some significant sources that are still undiscovered, is required due to the resistance of microorganisms to antibiotics. However, Bangladesh has only publicly disclosed a small number of works in this field [24]. The study aimed to identify the chemical constituents of both EOs and test the antimicrobial activity of two different types of Cosmos EOs.
Plant material
Two types of fresh leaf of Cosmos bipinnatus Cav. (cultivated) and Bidens ferulifolia (Jacq.) (wild) were collected in late December 2021 from the BCSIR Laboratories' experimental fields in Chittagong and identified by Dr. Md. Yousuf. Two specimens (A-110 and A-111) were put in the herbarium as proof at the BCSIR Laboratory in Chittagong.
Chemicals and media
Helium (purity>99.999%) and nitrogen gas (purity>99.999%) were purchased from Linde BD, Bangladesh. Standard antibacterial antibiotic ampicillin (20 μg/disc, BEXIMCO Pharma Bangladesh Ltd., Dhaka), antifungal nystatin, Dimethyl Sulfoxide (DMSO), acetone (GCMS grade), anhydrous sodium sulfate (analytical grade), Mueller-Hinton medium (Hi-media, India), Blood agar medium (Hi-media, India), Brain heart infusion (Hi-media, India), Sabouraud agar medium (Hi-media, India) were purchased from Sigma -Aldrich.
Extraction of EOs
For the extraction of EOs, hydrodistillation was used in accordance with the protocols outlined in earlier articles [25-28]. First, 50.0 g of fresh wild Cosmos leaves were added to a 1 L round-bottom flask with 500 ml of distilled water. This flask was then attached to a Clevenger-type apparatus with tap water for cooling. The EO was subsequently extracted after the leaves were hydro distilled for 4 hrs. The EO was then dried over anhydrous sodium sulfate to remove moisture. Physical qualities like color and odour were then noted. Second, the cultivated type of Cosmos leaf was also used to extract the EO using the same technique. The two EOs (wild and cultivated) were then sealed in separate vials and stored at 4°C until analysis.
Determination of the antibacterial activity of EO
The antibacterial effects of the cultivated EO was tested on ten potential pathogenic bacteria, including Bacillus subtilis BTCC 17, Pseudomonas aeruginosa (ICDDR, B), B. cereus BTCC 19, Escherichia coli ATCC 25922, Shigella dysenteriae AE 14396, S. sonnei CRL (ICDDR, B), Staphylococcus aureus ATCC 6538, S. paratyphi AE 14613, Salmonella typhi AE 14612, Vibrio cholerae AE 14748. The in vitro sensitivity of the bacteria to the test EOs was done by the disc diffusion method [24,28]. Mueller-Hinton medium was used for the culture of bacteria. Each experiment was repeated three times. All the results were compared with the standard antibacterial antibiotic, ampicillin. The strains were first screened for viability and purity on a blood agar medium before being stored on nutrient agar for use in bacterial susceptibility assays.
Antifungal activity of cultivated EO
The poisoned food method was used to test the antifungal activity of the EOs against six phytopathogenic fungi, viz., Alternaria alternate (Fr.) Kedissler., Colletotrichum corchori Ikata (Yoshida), Botryodiplodia theobromae pat., Fusarium equiseti (Corda) Saccc., Curvularia lunata (Wakker) Boedijin, and Macrophomina phaseolina (Maubl) Ashby of nystatin [24]. The subculture of fungi was conducted on Sabouraud agar medium. After 3-5 days of incubation, the fungus' linear mycelial growth was measured. The percentage inhibition of radial mycelial growth of the test fungus was calculated as follows:
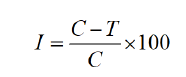
The percentage inhibition of radial mycelial growth of the test fungus was measured using I=percentage of inhibition; C=diameter of the fungal colony control; and T=diameter of the fungal colony in the treatment. All findings were compared with those observed using the standard antifungal drug nystatin (100 ppm). Each experiment also included a control set. Every experiment was carried out three times. Before use, the standard antifungal and antibacterial drugs and the EO were all separately dissolved in a specific amount of 30% Dimethyl Sulfoxide (DMSO).
Determination of the MIC, MBC, and MFC
To conduct the tests, plate discs were made, and antimicrobial assays were carried out using the agar dilution method, which was modified from the original procedure [24]. Each EO was independently diluted in MHA plus 0.5% Tween 80 on Petri dishes at 30°C to achieve comparable concentrations of 50 to 1000 ppm and 3.0% v/v for the MIC, MBC, and MFC experiments. The strains were cultivated in Brain Heart Infusion (BHI) for 18 to 24 h at 35°C, and standard suspensions were conducted in 0.85% sterile saline, using a scale of 0.5 of MacFarland as the target. A microdilution broth technique employing Mueller-Hinton medium was used to measure the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of EOs against 10 test bacteria. The MIC and Minimum Fungicidal Concentration (MFC) values of essential oils against 6 test fungi were also measured using microdilution broth procedures in a Sabouraud medium. Using a sterile multi-inoculator and suspensions standardized at 0.5 MacFarland, 16 strains were inoculated. A second dilution was then done on BHI to produce inoculum with a concentration of approximately 105–106 CFU/ml. The growth of the bacteria in Petri plates was evaluated, and the MIC values were noted for each strain after 18 to 24 h of incubation at 35°C. The MIC value for each tested bacterial strain was calculated by converting values from percent by volume to milliliters per liter using the density values of each oil.
GC-MS analysis of Cosmos EOs
The Gas Chromatography-Mass Spectrometry (GC-MS) analysis of both Cosmos (cultivated and wild) leaf EOs was carried out on a Shimadzu (QP 5050A series GC-MS system (Tokyo, Japan) equipped with an electron impact ionization (EI) on a GC-17A gas chromatograph with a mass spectrometer and fused silica capillary column (30 m × 2.5 mm; 0.25 µm film thickness; coated with DB-1 (J&W); stationary phase: 5% two phenyls, 95% two methyl polysiloxane coated; 40-350 amu) [25-27]. The column temperature was initially programmed to rise from 100°C (2 minutes) to 250°C at a rate of 3°C/min; the carrier gas was helium at a constant pressure of 90 kPa. The ionization energy was 70 eV with a scan time of 0.3 s. The management of the GC-MS system, parameter settings for GC and mass spectrometry, and data receipt and processing were performed using Shimadzu GC-MS Solution Version 4 software (Tokyo, Japan). The compounds were identified using two methods. One of the methods was based on a comparison of their mass spectra with data in the NIST Library (NIST 147 and NIST 27). The other was based on a comparison of their Retention Indices (RI) with those reported in the literature for the above column.
Statistical analysis
To compare the results of three or more independent testing treatments, the Kruskal-Wallis test was used. In multiple comparison tests where meaningful analysis p ≤ 0.001, is required, the Student-Newman-Keuls test is used to compare treatments.
EOs analysis by GC-MS
EOs is typically less dense than water, liquid, volatile, and rarely contains coloured lipid-soluble compounds. All plant tissues, including flowers, herbs, buds, leaves, fruits, roots, and others, are where they are synthesized. Additionally, there are numerous ways to extract these chemicals, including extraction, fermentation, steam distillation, and expression [29,30]. However, for food and pharmaceutical uses, steam distillation or cold pressing of the extract is preferred [29]. EOs are also complex combinations that include between 20 and 60 different components. They typically contain two main constituents in higher concentrations (20–70%) and several other minor compounds. Even though the biological activities are determined by the main constituents, overall, EO has more antibacterial activity than the individual pure constituents. Thus, the minor constituents can be critical in the antibacterial activity, indicating synergism is required [29,31]. The EOs from the plant Cosmos are particularly interesting in current studies. These EOs are well known for their antifungal, insecticidal, and antibacterial actions, which enable them to perform satisfactorily in protecting stored goods. The results, chemical compounds, and their percentages in the overall composition of each EO are presented in Table 1 (cultivated and wild). It is important to note that Bangladeshi-origin oils were used in the chemical analysis of the oils under investigation. The densities of all the oils tested were higher than 800 mg/ml, with wild EO having the highest density (1009 mg/ml) and cultivated EO having the lowest (820 mg/ml). The EOs was analyzed by GC–MS. In total, 36 and 16 constituents, respectively, representing 95.7% and 97.5 % of the total oils, were identified and quantified in the wild and cultivated plants (Table 1). In contrast, only 7 chemical constituents are common in both oils. (Z)-β-ocimene (28.76%), 2,6-dimethyl-1, 3,5-octatetraene, E.E (17.12%), germacrene D (19.19%), caryophyllene (5.40%), and elixene (4.64%) were found to be the major constituents in the wild plants. The main compounds in cultivated plants were (Z)-β-ocimene (48.29%), trans-3-Caren-2-ol (19.21%), sabinene (9.36%), germacrene D (6.10%) and b-pinene (4.18%). It was found that the quantity of the major compounds of Cosmos EO was increased in cultivated plants, e.g., (Z)-β-ocimene content increased by about 40% compared to its wild counterpart.
| Sr.No | Constituents | Ornamental (Cosmos bipinnatus) | Wild (Bidens ferulifolia) |
|---|---|---|---|
| 1 | a-thujene | -- | 1.95 |
| 2 | a-pinene | 0.46 | 0.05 |
| 3 | Sabinene | 9.36 | 0.89 |
| 4 | b-pinene | 4.18 | 0.07 |
| 5 | b-myrcene | -- | 0.71 |
| 6 | 3-carene | -- | 0.27 |
| 7 | b-trans-Ocimene | -- | 2.44 |
| 8 | (Z)-β-ocimene | 48.29 | 28.76 |
| 9 | 2-carene | -- | 0.13 |
| 10 | Carveol | -- | 1.06 |
| 11 | Geranyl nitrile | -- | 0.1 |
| 12 | 2,6-dimethyl-1, 3,5, -Octatetraene, E.E | 2.61 | 17.12 |
| 13 | 3-cyclohexene-1-metanol | -- | 0.11 |
| 14 | 4-terpineol | 2.46 | 0.18 |
| 15 | Azulene | -- | 0.08 |
| 16 | Terpinolene | -- | 0.49 |
| 17 | Bilagen | -- | 0.59 |
| 18 | Cyclohexane-1, 2-diol, 4(bicycl[2.21] hept-2-yl | -- | 0.13 |
| 19 | b-elemene | -- | 2.06 |
| 20 | Caryophyllene | -- | 5.4 |
| 21 | g-muurolene | -- | 0.62 |
| 22 | a-caryophyllene | -- | 1.5 |
| 23 | Germacrene D | 6.1 | 19.19 |
| 24 | Elixene | -- | 4.64 |
| 25 | d-cadinene | -- | 1.51 |
| 26 | Piepridine | -- | 0.08 |
| 27 | trans -nerolidol | -- | 0.22 |
| 28 | Caryophyllene oxide | -- | 2.1 |
| 29 | a-cyclocitral | -- | 0.26 |
| 30 | Ledol | -- | 0.44 |
| 31 | trans cadinol | -- | 1.53 |
| 32 | a-cadinol | -- | 1.21 |
| 33 | Juniper camphor | -- | 0.41 |
| 34 | Neoelovene(1), dihydro | -- | 0.54 |
| 35 | Octacosane | -- | 2.54 |
| 36 | b- pinene oxide | -- | 0.23 |
| 37 | Camphene | 0.23 | -- |
| 38 | b-phellandrene | 0.23 | -- |
| 39 | 1,4-cyclohexadiene,1-methyl-4-(1-methylethyl) | 0.79 | -- |
| 40 | 5,9-undecadien-2-one,6,10-dimethyl | 0.3 | -- |
| 41 | a-farnesene | 0.4 | -- |
| 42 | Trans-chrysanthenyl acetate | 0.25 | -- |
| 43 | 1,3,8-p-menthatriene | 3.04 | -- |
| 44 | 6,7-dimethyl-3,5,8,8a-tetrahydro-1H-2-benzopyran | 0.85 | -- |
| 45 | Trans-3-caren-2-ol | 19.21 | -- |
Table 1: GC-MS analysis of the EO composition of two species of Cosmos leaves (cultivated and wild).
GC-MS analysis of the EO composition of two species of Cosmos leaves (cultivated and wild).
Antimicrobial activity of cultivated EO
The cultivated EO was screened for its antibacterial activity against pathogenic bacteria and compared to that of the standard antibacterial antibiotics ampicillin. The results of the sensitivity test are shown in Table 2. Even at a concentration of 2 µg disc-1, an inhibition zone of 24 to 48 mm was observed. The inhibitions were found to be better against 10 test bacteria except for V. cholera, which was equal to standard ampicillin. The MIC value of the EO varied between 50 and 400 ppm against the test bacteria. The EO showed the lowest MIC value (50 ppm) against Pseudomonas aeruginosa. The MBC values of EOs against test microorganisms ranged from 200 to 400 ppm (Table 3). Six fungi were used to test the EO of Cosmos for antifungal activity, and the findings are shown in Table 4. All six test fungi showed complete and 100% inhibition of radial mycelial growth at a dose of 100 ppm, which was far higher than that of standard nystatin. The MIC values against the test fungus were observed to range from 50 to 300 ppm (Table 5). The lowest MIC values (50 ppm) against A. alternata, C. lunata, B. theobromae, and M. phaseolina were seen in the EO of Cosmos. The EOs MFC values were determined to range from 200 to 400 ppm (Table 5). Compared to all of the test fungi except C. corchori, the EO had the lowest MFC values (200 ppm).
| Antibacterial activity | |||||
|---|---|---|---|---|---|
| (Diameter of zone of inhibition in mm) | |||||
| Bacteria | Essential oil (μl/disc) | Ampicillin | |||
| 2 | 5 | 10 | 15 | (20 μg/disc) | |
| Gram-positive bacteria | |||||
| Bacillus cereus | 25 | 32 | 37 | 40 | 23 |
| Bacillus megaterium | 30 | 33 | 37 | 42 | 22 |
| Bacillus subtilis | 29 | 32 | 38 | 43 | 21 |
| Staphylococcus aureus | 32 | 35 | 38 | 40 | 24 |
| Gram-negative bacteria | |||||
| Escheriachia coli | 31 | 34 | 38 | 45 | 13 |
| Pseudomonas aeruginosa | 24 | 29 | 35 | 40 | 19 |
| Salmonella typhi | 28 | 32 | 39 | 43 | 30 |
| Shigella dysenteriae | 29 | 33 | 40 | 45 | 35 |
| Shigella sonnei | 25 | 30 | 36 | 43 | 30 |
| Vibrio cholerae | 31 | 38 | 43 | 48 | 24 |
Table 2: Antibacterial activity of EO of Cosmos leaf (cultivated).
| Bacteria | Bacterial growth in Mueller-Hinton broth medium (Essential oil concentration in ppm) | ||||||||
| 50 | 100 | 200 | 300 | 400 | 500 | 1000 | MIC (ppm) | MBC (ppm) | |
| Gram-positive bacteria | |||||||||
| Bacillus cereus | + | + | - | - | - | - | - | 200 | 300 |
| Bacillus subtilis | + | + | + | - | - | - | - | 300 | 400 |
| Bacillus megaterium | + | + | + | + | - | - | - | 400 | 500 |
| Staphylococcus aureus | + | + | - | - | - | - | - | 200 | 300 |
| Gram-negative bacteria | |||||||||
| Escheriachia coli | + | + | - | - | - | - | - | 200 | 300 |
| Pseudomonas aeruginosa | + | - | - | - | - | - | - | 100 | 200 |
| Salmonella typhi | + | - | - | - | - | - | - | 100 | 200 |
| Shigella sonnei | + | + | - | - | - | - | - | 200 | 300 |
| Shigella dysenteriae | + | + | - | - | - | - | - | 200 | 300 |
| Vibrio cholerae | + | + | + | + | + | - | - | 500 | 1000 |
Note: +: Growth present, -: Growth absent.
Table 3: MIC and MBC of leaf EO of Cosmos bipinnatus (cultivated) against 10 bacterial tests organisms.
| Antifungal activity (Inhibition of radial mycelial growth in percentage) | ||||||
| Fungus | EO concentration (ppm) | Nystatin (100 ppm) | ||||
| 100 | 250 | 500 | 750 | 1000 | - | |
| Alternaria alternate | 100 | 100 | 100 | 100 | 100 | 54 |
| Botryodiplodia theobromae | 100 | 100 | 100 | 100 | 100 | 70 |
| Colletotrichum corchori | 100 | 100 | 100 | 100 | 100 | 65 |
| Curvularia lunata | 100 | 100 | 100 | 100 | 100 | 71 |
| Fusarium equiseti | 100 | 100 | 100 | 100 | 100 | 42 |
| Macrophomina phaseolina | 100 | 100 | 100 | 100 | 100 | 69 |
Table 4: Antifungal activity of EO of Cosmos leaf (cultivated).
| Fungal growth in Sabouraud broth medium (EO concentration in ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fungus | 50 | 100 | 200 | 300 | 400 | 500 | MIC (ppm) | MFC (ppm) |
| Alternaria alternata | - | - | - | - | - | - | 50 | 100 |
| Botryodiplodia theobromae | - | - | - | - | - | - | 50 | 100 |
| Colletotrichum corchori | + | + | - | - | - | - | 200 | 300 |
| Curvularia lunata | - | - | - | - | - | - | 50 | 100 |
| Fusarium equiseti | - | - | - | - | - | - | 50 | 100 |
| Macrophomina phaseolina | + | - | - | - | - | - | 100 | 200 |
Note: +: Growth present, -: Growth absent.
Table 5: Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC) of EO from Cosmos leaf (cultivated) against 6 fungal test organisms.
The differences in EOs constituents between wild and cultivated types of the Cosmos have not been investigated previously. It has been reported that (E)-β-ocimene, germacrene-D, and sabinene were some major components in Cosmos bipinnatus EO [4]. Low EO content and high variability in EOs composition may restrict the industrial use of wild-growing Cosmos plants. This study showed Cosmos bipinnatus had better compatibility with cultivation in Chittagong, Bangladesh, which contributed to its uses as an alternative and natural product for flavour and medicine. According to the findings, the EO is a complex mixture of many different constituents, many of which are only present in small quantities. At this point, it is important to note that the chemical composition of cultivated EO varies widely. Despite the fact that (E)-β-ocimene is present in cultivated type in roughly double the amount found in wild type, it is the most important and prominent constituent in cultivated EO. EOs extracted from the plant contains volatile terpenes, hydrocarbons and are secondary metabolites with several biological activities [32-34]. However, its content was significantly higher in the EO obtained from the "cultivated" type (0.3%). When the results from the two types were compared, the EO isolated from cultivated plants had a significantly higher (Z)-β-ocimene (48.29%) than the EO separated from wild plants. The EO obtained from the "cultivated" type of Cosmos contained more (Z)-β-ocimene, whereas there was no statistical difference in the content of other constituents in the EO investigated in the wild.
The EOs MBC value against S. dysenteriae and V. cholerae was the lowest (300-400 ppm). The results are consistent with those of EOs from other plants that have been reported by other researchers [35-38]. Staphylococcus aureus had a MIC of 0.16 mg ml-1, while S. aureus was inhibited at 0.31 mg ml-1. Escherichia coli and Shigella sonnei were inhibited at 0.63 mg ml-1, while other Gram-negative bacteria, Proteus vulgaris, Enterobacter faecalis, and Shigella flexineri, were inhibited at 0.31 mg ml-1 in the EO of Cosmos caudatus [4,12]. Thus, the antibacterial assay showed that the EO had significant inhibitory effects against Gram-negative and Gram-positive bacteria, with the Gram-positive bacteria being more susceptible to the EO than the Gram-negative bacteria. Many researchers have noted that EOs from other plants also have comparable antifungal properties [39-41]. When tested against Alternaria alternata, Bortydiplodia theobromae, and Curvularia lunata, the EO exhibited the same lowest MIC and MFC values (100 ppm).
This study was funded by the R&D project of the Institute of Food Science and Technology (IFST), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka. The authors are grateful to IFST and BCSIR authority as well.
This research is being funded as part of BCSIR R&D project.
MNIB designed the study, performed the experiments with KAAS, and analyzed the data. MN, SA, MAH, MKUS, MMH, MASM, and MAAS gave suggestions, and wrote the introduction. All authors contributed to the drafting of the manuscript.
Citation: Bhuiyan MNI, Nahid M (2023) Comparison of the Chemical Composition and Antimicrobial Activity of Two Types of Cosmos (Cosmos bipinnatus Cav. and Bidens ferulifolia (Jacq.) Leaf Essential Oils Introduced in Bangladesh, J Chromatogr Sep Tech. 14:515
Received: 15-Apr-2023, Manuscript No. JCGST-23-23533; Editor assigned: 18-Apr-2023, Pre QC No. JCGST-23-23533(PQ); Reviewed: 05-May-2023, QC No. JCGST-23-23533; Revised: 15-May-2023, Manuscript No. JCGST-23-23533(R); Published: 23-May-2023 , DOI: 10.35248/2157-7064.23.14.515
Copyright: © 2023 Bhuiyan MNI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.