Organic Chemistry: Current Research
Open Access
ISSN: 2161-0401
ISSN: 2161-0401
Research Article - (2012) Volume 1, Issue 2
The complexation of KBr3 with acyclic poly (ethylene glycol)-400 was carried out in which the chain of polyether suitably wrapped around the cation in a host-guest manner. The resulting complex was found to be an efficient brominating agent for the selective regioselective monobromination of various aromatic compounds in excellent yields under mild reaction conditions. In another protocol, bromination was carried out by using the PEG-embedded KBr3 as a catalyst in the presence of hydrogen peroxide. The presence of hydrogen peroxide enhanced the reaction rates and provided selective bromination within very short reaction times.
Keywords: Host-guest complex; Bromination; Tribromide; PEG; Regioselective synthesis
After the synthesis of crown ethers and the discovery of their complexing properties toward alkali metal cations in 1967, the hostguest chemistry has developed rapidly and being used worldwide in various fields such as supra-molecular chemistry, biomimetic chemistry and materials science [1,2]. However, expensive nature and limited accessibility of the crown ethers make their utility limited. On the other hand, polyethylene glycols, referred as a “poor chemist’s crown ethers” are well known to be inexpensive, non-volatile, non-toxic and biodegradable, are also have the tendency to bind with alkali cations as crown ethers. Owing to the easy availability and cost effective nature, PEG’s, have widely been used in various applications [3,4]. Host-guest interactions of poly (ethylene glycols) to form inter polymer complexes through hydrogen bonding are well reported and have widely been used in drug-delivery systems [5,6]. However, host-guest complexation of PEG’s with organic reagents for organic reactions is not known so far. Monobromination of activated aromatic compounds such as phenols, aromatic amines etc. is an important synthetic transformation as these compounds are valuable intermediates in a variety of well-known reactions such as Wurtz-type condensation reactions, hydrolysis reactions, formation of Grignard reagents, and many other useful syntheses [7,8]. Further, they tend to undergo multiple bromination when treated with elemental bromine under the usual bromination conditions [9]. Therefore selective bromination of these compounds to mono-brominated products is a challenging task. A variety of improved protocols by using expensive transition-metal based catalysts [10], alkali metal halides associated with NaIO4 [11] or the combination of aqueous TBHP or H2O2 together with a hydrohalic acid [12] have also been reported for this transformation. However, these methods have certain limitations like use of expensive heavy transition metals, toxic/volatile chlorinated organic solvents and formation of polysubstituted and other side products. To encourage the development of sustainable synthetic methodologies, the use of eco-friendly reagents has become a subject of immense interest in present day chemistry [13,14]. In this regard, some ionic liquid tribromides (IL-Br3) have been reported for the bromination of aromatic substrates and synthesis of bromoesters from aromatic aldehydes [15]. The key drawbacks of these reagents are the use of expensive organic ammonium cations and difficult synthesis of these reagents. Ma et al. [16] studied the use of phosphonium based ionic reagents both polystyrene supported and ionic liquid based environmentally safe brominating agents for the bromination of unsaturated compounds. Le et al. [17] reported the regioselective monobromination of activated aromatics using 1-butyl-3-methylimidazolium tribromide ([Bmim]Br3) under solvent free condition. Very recently Zolfigol et al. [18] reported the {[K.18- Crown-6]Br3}n as a unique nanotube like structure as brominating agents. However, the expensive nature and limited accessibility of the crown ethers make this method of limited utility. This report led our interest towards utilizing the poly(ethylene)glycols as a cost-effective alternatives of expensive crown ethers for developing a new brominating agent. In continuation to our previous work on polyethylene glycols [19-23], we report herein a simple, economically affordable and efficient methodology for the selective mono-bromination of aromatic compounds under solvent-less conditions (Scheme 1).
Preparation of [K+PEG.Br3-] (2)
In a round-bottomed flask (100 ml) bromine (68.6 mmol, 10.8 g, 3.5 ml) was added to a 34 % aq. solution of KBr (25 g; prepared by adding the KBr (9 g) in water (135 g) to prepare the solution of potassium tribromide. Polyethylene glycol (PEG400; 27.4 g, 68.5 mmol) was added to the above solution and the mixture stirred for 5 h at room temperature. The dark orange-red colored solution was extracted with dichloromethane and the combined organic layer was dried over anhydrous sodium sulfate, concentrated under reduced pressure. The dark orange red viscous liquid was obtained in quantitative yield (44.5 g, 97 %). The loading of the KBr3 in the prepared reagent 2 was found to be 1.2 mmol/g.
General procedure for the bromination of aromatics
Method A: Stirred the mixture of the substrate (1 mmol) and reagent 2 (1 g, 1.2 mmol/g) at 30°C for the time as given in Table 1. At the end of the reaction (as analyzed by TLC), the reaction mixture was extracted with diethyl ether and the residual mixture was treated with bromine to regenerate the reagent 2. The combined organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. Conversion and selectivity of the products was determined by GC-MS and identity of the products was confirmed by comparing their physical constants, IR and NMR spectra with the authentic samples [24,25].
| Entry | Substrate | Product | Method | Time /min |
Convb/ Yieldc/ % |
|---|---|---|---|---|---|
| 1 | 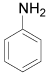 |
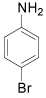 |
A B |
30 8 |
98/95 98/96 |
| 2 | 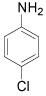 |
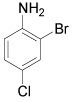 |
A B |
25 10 |
96/93 97/95 |
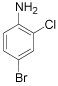
| 3 | 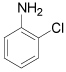 |
 |
A B |
30 10 |
97/94 98/96 |
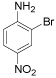
| 4 | 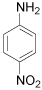 |
 |
A B |
35 10 |
94/88 95/90 |
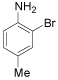
| 5 | 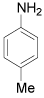 |
 |
A B |
20 5 |
95/92 97/93 |
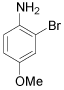
| 6 | 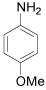 |
 |
A B |
25 10 |
99/95 99/94 |
| 7 | 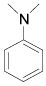 |
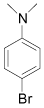 |
A B |
25 5 |
100/97 100/96 |
| 8 | 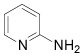 |
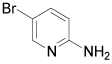 |
A B |
20 8 |
99/96 98/95 |
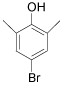
| 9 | 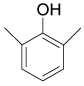 |
 |
A B |
35 10 |
97/94 99/96 |
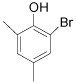
| 10 | 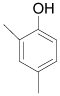 |
 |
A B |
30 10 |
92 97 |
| 11 | 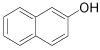 |
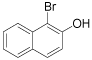 |
A B |
30 8 |
94/90 92/85 |
| 12 | 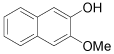 |
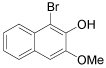 |
A B |
45 15 |
97/92 96/94 |
aReaction conditions: substrate (1 mmol), [K+PEG.Br3 -] (1 g) at 30°C
bConversion was determined by the GC-MS
cIsolated yields
Table 1: Bromination of aromatic compoundsa
Method B: The reaction mixture containing substrate (1 mmol) and catalytic amount of reagent 2 (10 mol%, 0.1 g) was charged with hydrogen peroxide (1.2 mmol) and the resulting mixture was stirred until the reaction was completed (as analyzed by TLC). At the end of the reaction, the product was isolated by extraction with diethyl ether and the resulting residual layer was reused for bromination after adding the fresh substrate and hydrogen peroxide.
Spectral data of selected compounds
p-Bromoaniline: (Table 1, entry 1): Brown solid; Mp (°C) [21] IR (KBr): 3472, 3379, 1615, 1489, 1282 cm-1; 1H NMR (300 MHz, CDCl3): 3.6 (b, 2H), 6.6 (d, 2H, J=6.5 Hz), 7.3(d, 2H, J= 6.9). MS (m/z) 173.
o-Bromo-p-toluidine: (Table 1, entry 5): Oil, IR: 3464, 3372, 3012, 2912, 1614 cm-1; 1H NMR (300 MHz, CDCl3): 2.2 (s, 3H), 3.9 (b, 2H), 6.6 (d, 1H, J=0.8Hz), 6.9 (d, 1H, J=2.4 Hz), 7.2 (s, 1H, J=8.0Hz). MS m/z 187.
5-Bromo-2-aminopyridine: (Table 1, entry 8) IR: 3402, 3186, 1650, 1569, 1485 cm-1; 1H NMR (300 MHz, DMSO): 6.8 (b, 2H, J=1 Hz), 8.2 (s, 2H,); 13C NMR (300 MHz, DMSO): 104.2, 155.2, 159.0, 161.7; MS m/z 175.
1-Bromo-2-napthol (Table 1, entry 11): 1H NMR (300 MHz, CDCl3): δ 5.7 (b, 1H), 7.4-7.6 (m, 4 H), 7.8-7.9 (m, 2H). MS: m/z 224.
1-Bromo-3-methoxy-2-napthol (Table 1, entry 12): 1H NMR (300 MHz, CDCl3): δ 3.4 (s, 3H), 5.6-5.7 (b, 1H), 7.3-7.5 (m, 4 H), 7.6 (s, 1H). 13C NMR (300 MHz, CDCl3): 105.3, 119.0, 122.5, 126.1, 127.0, 133.4, 145.0. MS: m/z 254
For this purpose, we synthesized the potassium tribromide (KBr3) by the addition of equimolar amounts of aq. solution KBr and Br2 as following the literature procedure [26]. The prepared tribromide was stirred for 5 h in PEG400 at 30°C, resulted in the formation of dark red-orange viscous oil which was dried under vacuum before use. The loading of the reagent 2 was found to be 1.2 mmol/g as determined by elemental analysis. The X-ray diffractogram of [K+PEG. Br3-] in the liquid phase at room temperature was not very clear and showed a broad low-angle peak between 19.5-22°. Unfortunately, we could not get any information regarding the structure of reagent 2. However, the prepared reagent was dark red in color and its red color was slowly lost as bromination occurred during the reaction. After completion of the bromination reaction, no bromine could be detected in the reaction mixture as analyzed by UV-Vis spectroscopy. Following the developed methodology, a variety of aromatic compounds were reacted with [K+PEG. Br3-] 2 (Method A) at room temperature under solvent free conditions. All the substrates were selectively converted to the corresponding monobrominated products in excellent yields. The results of these experiments are presented in Table 1. In contrast to the elemental bromine, the present method is advantageous as it is easy in handling and provides a highly efficacious approach for achieving the mono-brominated aromatic compounds selectively in higher yields. After completion of the reaction the brominated product was extracted with diethyl ether and the resulting residue containing [PEG.KBr] was treated with molecular bromine to regenerate the [K+PEG. Br3-].
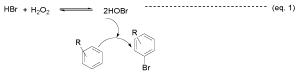
In another protocol (Method B) the bromination of the aromatic compounds was taken place in the presence of aq. hydrogen peroxide by using [K+PEG. Br3-] 2 as catalyst. The results of these experiments are summarized in Table 1. After completion of the reaction, the product was separated by extraction with diethyl ether and the resulting residue could be used as such for the bromination reaction after addition of the fresh hydrogen peroxide and substrate. The presence of hydrogen peroxide enhanced the reaction rates significantly and bromination of the substrates was occurred within very short reaction times. The significant effect of hydrogen peroxide is probably due to the formation of hypobromous acid (HOBr) [27] in the system, which provides the active brominating species (Br+) and allows the instant bromination (equation 1).
The develop method is advantageous in number of ways for example, the PEG-embedded KBr3 reagent is readily formed by using the cost-effective reagents such as KBr, provides selective synthesis of mono-brominated products, reagent can easily be regenerated and recycled without loss in activity, solvent free conditions and moreover easy work-up of the products. Next, the effect of the various solvents was also studied for the bromination of aniline under described reaction conditions. Among the different solvents studied water was found to be better, whereas, solvent free condition was found to be best, making the method more advantageous in terms of product isolation as well as environmental viewpoints. The selectivity of the reaction was found to highly dependent upon the reaction temperature.
In conclusion, we have developed a new, simple, cost effective and solvent-less methodology for the selective bromination of the aromatics by using the complex [K+PEG. Br3-] prepared by the KBr3 with acyclic poly(ethylene)glycol-400 PEG, in which the chain of polyether suitably wrapped around the cation in a host-guest manner. This reagent is advantageous in a number of other respects such as it is readily formed, safer and easy in handling. Further, the reactions can be conducted at ambient temperatures and afforded high yields of the desired products.
We kindly acknowledge the Director, IIP for his kind permission to publish these results and SV is thankful to CSIR, New Delhi for the research fellowship.