
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 3
Anti-Vascular Endothelial Growth Factor (VEGF) therapy is the leading treatment strategy for Age-related Macular Degeneration (AMD). Although this treatment generally improves VA of the affected patients, its prolonged application can lead to serious complications.
Purpose: The purpose of our study is to outline the possible long term complications of anti-VEGF treatment in AMD patients and to outline eventual ways to avoid some of them.
Methods: In our prospective study 42 patients with wet AMD were enrolled. They all underwent a complete ophthalmological examination including Visual Acuity (VA), fundus photography, structural Optical Coherence Tomography (OCT) (Revue, Optovue) and OCT-A (Angiophlex, Zeiss). All of the patients were treated with aflibercept (Eylea)-in the Treat and Extend regiment for a period of 2 years. The mean number of injections was 15 ± 2. All patients were evaluated for possible complications after the 2 year period.
Results: The long term complications we encounter can be summarized in percentage % as follows:
• Tachyphylaxis to the treatment drug-20%
• RPE Tear-10 %
• Retinal fibrosis and scar formation-32%-35%
• Retinal atrophy 25%
In 20% of the patients a tachyphylaxis developed after the first year, which leads to lower effect of the drug on the course of treatment. Discontinuation of the treatment or change to another drug helped against that complication. In 10% of the cases Retinal Pigment Epithelial (RPE) tear developed usually in cases with RPE detachment, large in area and height. Retinal fibrosis was the most serious complication. In our study it developed mainly in patients with lower VA at baseline, with macular hemorrhages or intraretinal cysts. Retinal fibrosis developed usually after the 8 intravitreal injection. In 25% retinal atrophy developed
Conclusion: Complications of anti-VEGF therapy are relatively rare but leading to devastating results. The stronger the action of the anti-VEGF drug the more frequent the complications, especially fibrosis. Early recognition of the risk factors such as low vision at the beginning. Intraretinal cysts, macular hemorrhages and large area of CNV are a prerequisite for a successful prophylaxis of the possible complication.
Complications; AMD; RPE tear; Retinal fibrosis; Anti-VEGF
Age-related Macular Degeneration (AMD) is a disease with high social impact due to it significant sight threatening complications. The disease can be found in two forms "dry" AMD with slow, progression to geographical atrophy and exudative or "wet" form, with fast development of subretinal neovascular membranes. Epidemiological studies show that AMD occurs in about 11% of patients over 65 years of age and its incidence increases with age [1-3].
Dry form of AMD is found in 85% of the cases and only in 15% the exudative form of the disease. However these 15 % are responsible for the visual impairment and blindness due to AMD [4]. In the United States and the United Kingdom, age-related macular degeneration is the cause of 50% of cases of blindness, and its incidence increases with life expectancy. It is estimated that by 2025 the number of cases would double [5-7]. AMD is characterized with progressive visual impairment that significantly disturbs the quality of life of patients. By 2050 the number of patients worldwide will reach 288 million, which is twice as many as those with Alzheimer's disease in the world [8-10].
The pathophysiological mechanisms of the disease are still subject of discussions and various theories. It is generally accepted that many factors affect the aging process by disrupting the functional integrity of the system photoreceptor cells, retinal pigment epithelium and Bruch's membrane [1,8]. According to one of the most accepted theories that of aging, pathological changes are associated with degenerative changes in retinal pigment epithelial cells –PPE [11]. As a result, these cells cannot perform their phagocytic function, and undigested waste products accumulate between RPE and the Bruch's membrane known as basal laminar deposits, which are visualized ophthalmoscopically as druses [11,12]. Predispositions are created for new-vessel formations from choriocapillaris in the subretinal space, with subsequent detachment of RPE and damage to vision. This theory, although quite commonly accepted, is not the only one discussed. Factors such as oxidative stress, chronic inflammation, ischemia, etc. are also important.
Until recently, the wet form of AMD caused irreversible vision loss in two-thirds of the examinde patients [1,5]. The use of anti-VEGF drugs however made it possible to diminish by half the number of patients with blindness in the United States and maintain good visual acuity [13,14]. The application of anti-VEGF drugs is a selective, highly effective therapy with relatively few side effects [10,15]. Thus a high concentration of the substance is inserted in the vitrous. Anti-VEGF drugs generally block one or all isoforms of the vasoproliferative growth factor VEGF and prevent the formation of new-vessels in the exudative form of AMD [10,13,15].
The VEGF family of proteins includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and VEGF-E, and placental growth factor [10,15]. The actions of VEGF family members are mediated by the activation of tyrosine-kinase receptors. Current anti-VEGF agents, including ranibizumab (Lucentis; Genentech, South San Francisco, California), bevacizumab (Avastin; Genentech) and aflibercept (Eylea; Regeneron, Tarrytown, New York) demonstrated meaningful improvement in vision. Ranibizumab is a humanized IgG1 antibody fragment against VEGF-A, while bevacizumab is a recombinant humanized monoclonal IgG1 antibody against VEGF-A. Aflibercept is a recombinant protein created by fusing the second immunoglobulin domain of human VEGF receptor 1 with the third domain of human VEGF receptor 2, which in turn is fused to the constant region of human IgG1 [13,16,17]. All the know anti-VEGF drugs have similar efficacy. This is confirmed by various studies with comparative data such as CATT, IVAN, VIEW1 and VIEW2 etc. All the protocols turned out to have very similar efficacy in terms of visual acuity at the end of one and two years [18,19].
Although anti-VEGF therapy improved the outcome of wet AMD immensely, it can inflict also some serious complications. One of the concerns in the long-term use of anti-VEGF agents is the possibilities of retinal fibrosis and geographic atrophy. Data from the CATT and HARBOR trials suggests that monthly dosing seems to increase the rate of development of macular fibrosis and atrophy, with no consistent difference between the anti-VEGF drugs [19]. However, all these questions are to be further analyzed and discussed.
Purpose
The purpose of our study is to outline the possible long term complications we observed in the anti-VEGF treatment of AMD patients. It is our aim to discuss and propose possible ways to detect and avoid some of them.
In our prospective study 42 patients with wet AMD were enrolled. 23 of them were male and 19 female. AMD in both eyes was found in 50% of all subjects (Table 1).
| Gender | AMD in both eyes | Mean | χ2 | ddf | p | ||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| Мale | N | 13 | 10 | 23 | 8,11 | 1 | 0,004 |
| % | 55,1% | 37,8% | 47,6% | ||||
| Female | N | 8 | 11 | 19 | |||
| % | 44,9% | 62,2% | 52,4% | ||||
| All | N | 21 | 21 | 42 | |||
| % | 100,0% | 100,0% | 100,0% | ||||
Table 1: Distribution of patients according to gender and affection of one or both eyes from AMD.
They all underwent a complete ophthalmological examination including VA, fundus photography, structural OCT (Revue, Optovue) and OCT-A (Angiophlex, Zeiss). All of the patients were later treated with aflibercept (Eylea) in the Treat and Extend regiment for a period of 2 years. Eylea (2 mg/0.05 ml) was first injected monthly for the period of 3 months and then on 56 days for the remaining period of two years. The mean number of injections was 15 ± 2. All patients were then evaluated with OCT for possible complications.
Optical coherence tomography
OCT is a modern technology for diagnosing retinal and optic nerve diseases. This is a relatively new methodology for non-invasive diagnosis, which produces "optical sections" of the examined tissue, giving information equal to that of histological specimens. For this reason, the method has been called "optical eye biopsy". The principle of operation of OCT is similar to B-sonograpy, but instead of ultrasound a beam of light from a low-coherence laser source with a wavelength of 840 nm is used [20]. For the purposes of the present study, we developed a protocol of programs in the OCT, which allowed us the most accurate and detailed assessment of the data and tracking of changes over time. In the recent years the rapid development of OCT technology lead to the establishment of a new diagnostic tool OCT-A.
OCT-A is also a non-invasive method which is used for studying retina‘s bloodstream and that of choroid. OCT-A segments the retina and provides information on vascular microcirculation in 3 zones superficial retinal plexus (vascular network between ILM and IPL), deep retinal plexus (vascular network between IPL and OPL.) and retinal avascular zone. In our studies we used OCT-A AngioPlex, Zeiss.
In the statistical analysis the following methods were used:
• Test of Normality (test of Normality Kolmagorov-Smirnov) of the distribution of a quantitative variable.
• Variation analysis of quantitative variables.
• Interval estimates of averages (95% of the confidence interval)
• A variance analysis of a quantitative variable in the presence of one factor (independent variable) with more than two categories was performed by ANOVA. Analysis of variance allows us to test the hypothesis that several means are equal. The null hypothesis (all means are equal) is rejected if p <0.05. The specialized statistical package Statistical Package for the Social Sciences (SPSS), version 16.0 was used to process the survey data.
In our study we observed various complications after anti-VEGF therapy, which we divided into two major groups.
Complications due to the injection itself:
• Endophthalmitis
• Retinal detachment
• Increased IOP
• Hemorrhages subconjunctival, retinal
• Systemic side effects
Complications due to the clinical course of AMD:
• Resistance to therapy
• RPE rupture
• Retinal atrophy
• Fibrosis
Complications due to the procedure of intravital injection were less than 3%. Of these, we mainly observed subconjunctival hemorrhages and increased IOP. In only one patient endophthalmitis developed. We did not observe patients with retinal detachment or intra-retinal hemorrhages. Subconjunctival hemorrhages were quite common in about 10% of all patients treated with intravenous drugs. They were more common in patients on anticoagulant therapy. These hemorrhages were usually benign and disappeared after about 7 days.
Elevated IOP after intravitreal injection was a common complication. It was generally observed in patients suspected for or with existing glaucoma, and occurred within 30 minutes after injection. IOP variations were in the range of 23-26 mm Hg and none of our patients had a pressure above 30. No therapeutic paracentesis was required. In 5 of the observed patients, a permanent increase in IOP and need of anti-glaucoma therapy was reported. Of greater importance for our study were the complications due to the further course of development of AMD. In the long run, we observed the following complications expressed in %:
• Tachyphylaxis to the drug substance 20%
• Tears of RPE-10%
• Fibrosis and scarring-45%
• Retinal atrophy 25%
Non-response to therapy and tachyphylaxis
Although relatively rare, we had cases of non-response to therapy or tachyphylaxis (after initial good responce than lack of therapeutic result). Often in these cases after the 3rd saturating dose the condition remained unchanged or even the fluid in the retina increased. This was usually seen in 27% of the followed cases (Table 2).
| Responsiveness to anti-VEGF treatment | Mean | χ2 | df | p | |
|---|---|---|---|---|---|
| No | N | 11 | 9,93 | 1 | 0,002 |
| % | 27,0% | ||||
| Yes | N | 31 | |||
| % | 73,0% | ||||
| All | N | 42 | |||
| % | 100,0% |
Table 2: Chi-Square Tests showing the percentage of patients non-responsive or tachyphylaxic to anti-VEGF therapy.
In tachyphylactic patients lack of reduction of the retinal fluid or the size of the detachment of the RPE was seen which remained for months. Furthermore in some of them additional fluid appeared. It is our notion that cases in which the amount of fluid in the retina remains the same for 4 weeks after the last injection can be considered tachyphylactic to treatment agent (Figure 1).
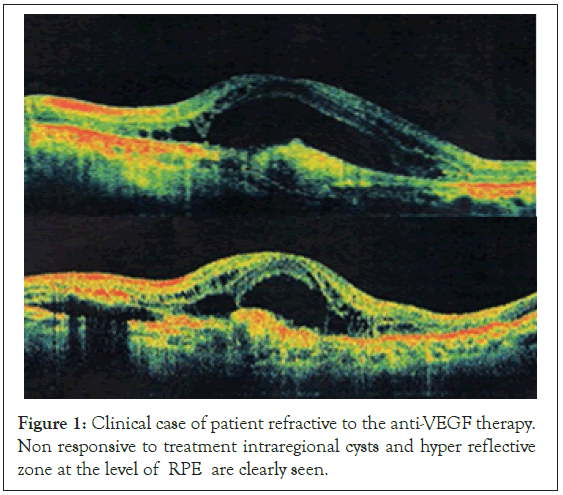
Figure 1: Clinical case of patient refractive to the anti-VEGF therapy. Non responsive to treatment intraregional cysts and hyper reflective zone at the level of RPE are clearly seen.
Our study patients refractory to treatment or with tachyphylaxis were usually those with:
• chronic intraretinal cysts and cystoid edema of the macula.
• with pre-existing hyperreflexive deposits, especially centrally lockated.
• with hyperreflective detachment of RPE, with a big size and height.
In addition to patients with refractory intraretin edema, those with treatment-resistant RPE detachment were also a particular problem. In tachyphylactic patients, treatment should be stopped and change of medication with other agent should be done.
RPE tear
Another serious complication, we observed in our group of patients was RPE tears. The tear may be part of the natural course of the disease, or may be provoked by ongoing anti-VEGF therapy. Spontaneous tear of vascularized RPE detachments occur in about 10% of cases [19]. That was the percentage we encountered in our study. The higher percentage is associated with long-term use of anti-VEGF drugs. As early as 2006, there were cases of RPE tear after administration of Avastin. Anti-VEGF drugs have been shown to cause contraction of neovascular membranes in vascularized detachments and this is the main reason why they accelerate the process of RPE tear
RPE tear is associated with a sharp decline in visual acuity. In the sample we observed we had 5 cases of RPE tear. In one case, it was a 65-year-old patient with severe serous detachment of RPE, with significant size and height. The patient was treated with Eylea for 1 year 8 injections. After the 8th application, visual acuity suddenly deteriorated from 0.2 to 0.01, and on the OCT and FA RPE tear has been observed (Figure 2).
Figure 2: Clinical case of RPE tear after anti-VEGF therapy, the site of the tear is clearly seen on the FA.
In all of the patients with RPE tear we observed hyperreflective deposits on the OCT, wich generally increases the risk of rupture. RPE cracks visible on the FA were also found. We observed those in 2 of the patients with RPE tear. According to our study, RPE detachments that are relatively recent are at greater risk of developing RPE tear than the old ones. In all of our cases RPE detachments were not more than 2 months of duration. Most likely that is due to the presence of a young neovascular membrane that is easier to contract. Conversely, older detachments are usually fibrovascular, with less active neovascular membranes and fewer opportunities for contractile forces. In all of our cases of RPE tear we stopped the intravitreal injections. In one of them because of activation of the disease we switched to 0.5 mg Lucentis and closely followed the patient. In any case, monitoring of high-risk patients is very important.
RPE fibrosis
Macular fibrosis was the worst complication we had after anti –VEGF therapy. Macular fibrosis is the last stage of AMD. The condition was described by Pagenstecher, 1875 and leads to the destruction of RPE, photoreceptors and severe damage to visual acuity. In our study, we observed fibrosis in 32% of the patients. On ophthalmoscopy, fibrous areas were observed as well-defined yellowish-white lesions at the retinal level. OCT diagnosis of the patients with fibrosis showed subfoveolar hyperreflectiveness, with clearly defined boundaries.
The main risk factors for the development of fibrosis that we observed in our study are the following:
• Presence of macular hemorrhage
• RAP or PCV
• Presence of refractory intraretinal cysts
Most patients with fibrosis were very much alike the following clinical case (Figure 3). A 64-year-old woman with refractory to treatment RPE detachment was treated with Eylea. During treatment with the initial 3 loading doses, a rapid decrease of the detachment was observed. After 1 year of treatment, however a recurrence of the disease and accumulation of hyper reflective material subfoveolar was seen. In the second year, subsequent fibrosis developed with a decrease of visual acuity. Our results point out that patients with RPE detachment combined with intraretinal cysts are at the highest risk of developing fibrosis.
Figure 3: Clinical case of fibrosis development after the treatment with Eylea. Although at the beginning a possitive result was acheived, in the long term a fibrous tissue developed.
Usually our patients had initial good outcome but later on with the disease recurrences fibrous tissue developed. Fibrosis most commonly affected those with low initial visual acuity, thin choroid especially subfoveolar and after average number of 7-8 intravitreal injections.
Complications after anti-VEGF therapy are relatively rare, but always associated with very serious visual impairment. The stronger the effect of the anti-VEGF drug, the more common the complications, especially retinal fibrosis. Aflibercept have much higher affinity for anti-VEGF factors 140 times greater than ranimizumab, its vitreous half-life is more than 1 month, which surpasses all other drugs in this group [15,18]. Alfibercept is a strong acting anti-VEGF drug and it is not unusual to cause complications. All the comlications we encounter are described in different extend in other studies such as Minimally classic/occult trial of the Anti-VEGF Antibody Ranibizumab in the treatment of Neovascular AMD (MARINA) and Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) [21-23]. They report of 25% refractory to tretment cases after application of ranibizumab and 40% refractory cases after tretment with bevacizumab [22]. At the same time, other authors [23,24] showed data on 25% refractory treatment in patients treated with Eylea. They point out that patients with PCV are most often refractory to therapy. Our study shows similar percentage of patients refractive to anti-VEGF of about 27% mostly in those with intraretinal cysts.
At the same time, patients with resistant to treatment intraretinal cysts showed much higher risk of fibrosis (3.3 times more often) and of atrophy (4.2 times more often) than in other cases of AMD [24,25]. Also, such patients were at higher risk of vision loss of about 10 letters or more (p=0.018). Our results correspond to yhe general findings that refractory cases are related to presence of large amount of intraretinal fluid. However in some cases good anatomic result is reported but not significant change in the VA is seen. RPE tear as a complication of intravitreal therapy in AMD is reported in the literature to be between 0.06 and 27% and that is several times higher than the spontaneous tears in the natural couse of the disease. Thus it is clear that anti-VEGF therapy increases the incidence of that serious complication.
In our study we reported that detachments with a height of 350 μm on the OCT have a higher risk of tearing. This was confirmed by He et al. [26,27], who reported RPE rupture at high of 400 μm. He et al. [26] prove statistically that a height of RPE of 580 μm necessarily leads to RPE tear. Similarly, others describe a height of 550 for a high risk factor for the development of RPE tear [27,28]. Another significant risk factor we observed is the time of RPE detachment. According to our results newly formed RPE detachments are at greater risk of that complication probably due to the presence of a young neovascular membrane that is easier to contract. He et al. [26] confirm our results and report an inverse relationship between the duration of RPE detachment and its rupture. In the presence of RPE tear, it is mandatory to make an OCT assessment and to stop the administration of the drug for some time. If not possible, change of medication and reduction of the dose is recommended [28].
Fibrosis is one of the most devastating complications of anti- VEGF therapy. In general, lots of authors report that anti-VEGF drugs after long-term use over 2 years, lead to the development of fibrosis. It develops spontaneously as a natural course of the disease, in 39% of cases, but after the introduction of intravitreal injections, this percentage increased to 55% [1,29]. Fibrosis is associated with the growth of connective tissue and is characterized by deposition of elements of the extracellular matrix in the process of tissue repair [30]. In tissue recovery activation of inflammatory cells and fibroblasts takes place. In the eye, activated fibroblasts are myofibroblasts and they synthesize proteins such as fibronectin, laminin and glycosaminoglycans. When the damage reoccurs or the inflammation is chronical, the recovery process is uncontrollable and pathological excessive fibrosis develops [31,32].
Risk factors for fibrous development can be different
Many authors in a number of genetic studies have shown [29,33] that there are genetic phenotypes that are the cause of poor response to therapy and predetermine the development of fibrosis. Ho et al. [3,33] reported that low levels of 25-hydroxyvitamin D in patients with AMD are the cause of more frequent fibrosis in these patients. The CFH Y402H CC genotype is thought to be associated with poor prognosis and resistance to anti-VEGF therapy. The authors prove that patients with this genotype are much more likely to develop fibrosis.
Other risk factors for fibrosis which we observed are:
• initially low visual acuity
• macular hemorrhage
• RAP presence of subretinal hyperreflective material visible on OCT-sign of poor prognosis and development of subretin fibrosis.
Development of subretinal fibrosis is also much more common in patients with RAP, in whom recurrences of the disease are frequent. Preventing fibrosis is quite difficult to achieve. It is always advisible to look for the predisposing factors and closely monitor patients in risk groups.
Complications of anti-VEGF therapy are relatively rare but leading to devastating results. The stronger the action of the anti-VEGF drug the more frequent the complications, especially fibrosis. Early recognition of the risk factors such as low vision at the beginning. Intraretinal cysts, macular hemorrhages and large area of CNV are a prerequisite for a successful prophylaxis of the possible complication.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Vidinova C, Antonova D, Vidinov K (2022) Complications after Anti-Vascular Endothelial Growth Factor Treatment of Patients with Wet Age- Related Macular Degeneration. J Clin Trials. 12:498.
Received: 16-May-2022, Manuscript No. JCTR-22-17504; Editor assigned: 18-May-2022, Pre QC No. JCTR-22-17504 (PQ); Reviewed: 01-Jun-2022, QC No. JCTR-22-17504; Revised: 08-Jun-2022, Manuscript No. JCTR-22-17504 (R); Published: 15-Jun-2022 , DOI: 10.35248/2167-0870.22.12.498
Copyright: © 2022 Vidinova C, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.