Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2017) Volume 7, Issue 3
The alkaloid and non-alkaloid fractions of purple sweet potato (Ipomoea batatas) written as AEIB and NEIB respectively were used as inhibitors in the corrosion reaction of mild steel in 1.0 M H2SO4 solutions. The corrosion rate decreased with increase in the concentration of the AEIB and NEIB as evidenced in the experimental results obtained from the gravimetric and gasometric techniques. This proved that the AEIB and NEIB are effective inhibitors of mild steel corrosion. Also, the experimentally obtained data fitted well into the Temkin isotherm while the computed thermodynamic parameter values showed that the inhibitors effectively reduced the rate of metal dissolution by becoming physically adsorbed on the mild steel surface.
<Keywords: Alkaloid; Non-Alkaloid; Ipomoea batatas; Gravimetry; Gasometry; Temkin isotherm
Corrosion is an electrochemical process which causes gradual deterioration to metallic structures through anodic dissolution [1]. This phenomenon is chemically induced on a metal and leads to destruction of properties and construction failure. Mild steel is more prone to corrosion when exposed to the action of acids or bases. The use of inhibitors during acid pickling, acid descaling, oil well acidizing are practical methods for corrosion control in acidic media [2]. There are various methods of corrosion control and prevention; popular among them is the use of corrosion inhibitors. Corrosion inhibitors are substances which when added in small concentrations to corrosive media decrease or prevent the reaction of the metal with the media [3]. Most of the effective inhibitors are used to contain hetero atoms such as O, N, and S with multiple bonds in their molecules through which they are adsorbed on the metal surface [4]. Some synthetic compounds showed good anticorrosive properties, but most of them were found to be toxic to man and a threat to the environment, hence the need to discover more eco-friendly inhibitors [5]. Extracts of many plants have been investigated and reported by several authors to be eco-friendly inhibitors of the metal dissolution in various acidic and alkaline media. Plant extracts have been generally accepted because they are eco-friendly, inexpensive, readily available and renewable sources of materials [6]. Plant extracts including their leaves, roots, tuber, stems and bark have been widely examined by several authors. Ipomoea batatas leaf has been reported to contain alkaloids, flavonoids, saponins, steroids, glycosides, tannins, anthocyanins [7]. It also shows antioxidant, antimicrobial, antimutagenic and antidiabetic effects [8]. In a recent study by [9] Eugenol and Indole-3-aldehyde were some of the several chemical compounds isolated from the leaves of Ipomoea batatas; which form the basis of the computational study. The present study intends to investigate the inhibition potentials of the leaves extract on mild steel corrosion in sulphuric acid media.
Sample preparation
The leaves of Ipomoea batatas used were obtained from a bush in the swampy areas of Calabar. They were oven dried at a temperature of 323 K and crushed into powder. 100 g of the leaves powder was extracted using 250 cm3 of 96% ethanol in a soxhlet extractor. The ethanol extract was separated into the alkaloid and non-alkaloid fractions in a separating funnel. The stock solution of 10 g/L concentration was prepared and 0.1, 0.2, 0.3, 0.4 and 0.5 g/L of the inhibitor solutions were derived. In a separating funnel, 10 g of the dried sample of ethanol extract was dissolved using 100 ml of chloroform and 100 ml of 0.1 M sulphuric acid and shaken thoroughly until a homogenous mixture was obtained. The mixture was allowed to stand for 24 hours to obtain a clear separation. The tailing fraction was collected from the tap and labeled as the non-alkaloid fraction of the extract. 100 ml of Ammonia was added to the fraction left in the separating funnel in order to basify the remaining mixture and shaken together for thorough mixing and after 6 hours, a clear liquid separated out leaving another mixture which was collected and used as the alkaloid extract.
Mild steel coupons of dimensions: 4.0 × 4.0 × 0.1 cm and 4.0 × 2.0 × 0.1 cm were used for gravimetric and gasometric analysis respectively. In gravimetric measurements, Mild steel coupons were weighed before total immersion in a 250 ml beaker containing test solutions of different concentrations, being suspended by suitable hooks at 1 cm below the solution surface and retrieved after 1 hour, scrubbed with brush under running distilled water, dried as before and weighed. The difference in weight of the coupons before and after immersion in different test solutions was taken as the weight loss. The inhibition efficiency of the inhibitor (IE%) of the inhibitors was calculated using equations 1 from the corrosion rate values obtained from the plot of weight loss against time.
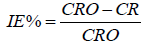 (1)
(1)
CR and CR0 are the corrosion rate in the presence and absence of inhibitors respectively. A 100 ml test solution poured into a reaction vessel used for the gasometry. The metal coupon was introduced into the reaction vessel and corked. The decrease in volume of the paraffin oil in the burette due to hydrogen gas evolution was noted after every one minutes till the last volume of paraffin oil in the burette after 60 minutes. The volume of hydrogen gas evolved was used to evaluate inhibition efficiency of the test inhibitors using equation 2.
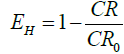 (2)
(2)
Where CR and CR0 represent the corrosion rate derived from total volume of gas evolved in the presence and absence of test inhibitors respectively [3].
Weight loss results
Mild steel was found to corrode at an appreciable rate in 1 M H2SO4 solution. Results obtained clearly show that extracts of Ipomoea batatas leaves effectively retard the corrosion rate of mild steel in 1 M H2SO4 solution, indicating inhibition of the corrosion reaction. The weight loss data reveal the changes in the weights of the metal coupons with time and increase in the concentration of AEIB, NEIB led to improved inhibition efficiency as shown in Figures 1a and 1b. The extract inhibits corrosion by controlling the redox reactions [10]. The optimum inhibition efficiency obtained at 85.2% and 72.5% for AEIB and NEIB extracts respectively at 303 K temperature with 0.5 g/L inhibitor concentration (Table 1).
| AEIB | NEIB | |||||
|---|---|---|---|---|---|---|
| Conc. (g/L) | Corr. Rate (mg/cm2/hr) | θ | Inhibition Efficiency | Corr. Rate (mg/cm2/hr) | Inhibition Efficiency | |
| Blank | 5.110 | - | - | 5.110 | - | - |
| 0.1 | 3.219 | 0.370 | 37.0 | 3.609 | 0.294 | 29.4 |
| 0.2 | 2.256 | 0.558 | 55.8 | 2.977 | 0.417 | 41.7 |
| 0.3 | 1.456 | 0.715 | 71.5 | 2.434 | 0.524 | 52.4 |
| 0.4 | 0.992 | 0.806 | 80.6 | 1.590 | 0.689 | 68.9 |
| 0.5 | 0.755 | 0.852 | 85.2 | 1.407 | 0.725 | 72.5 |
Table 1: Calculated values of corrosion rates, surface coverage and inhibition efficiency for 104 extracts of Ipomoea batatas leave on Mild steel in 1 M H2SO4 at 303K.
Temperature dependent result
The continuous decrease in inhibition efficiency with increasing temperature (Table 2) signifies that the inhibitors function effectively at lower temperatures. It also indicates that the time lag for the adsorption and desorption of the inhibitor molecules on Mild steel coupon surfaces become shorter at higher temperature. Table 2 revealed that the most remarkable reduction in the volume of hydrogen evolved was observed for AEIB at 0.5 g/L with inhibition efficiency of 94.9%. Also, the inhibition efficiency obtained from the gasometry measurements also increased with increase in inhibitor concentration. Hence, the results obtained from the gasometric and gravimetric experiments are in good agreement.
| AEIB | NEIB | |||||
|---|---|---|---|---|---|---|
| Inhibitor Conc. (g/L) | 303K | 313K | 323K | 303K | 313K | 323K |
| Blank | - | - | - | - | - | - |
| 0.1 | 74.8 | 71.9 | 49.1 | 59.8 | 32.5 | 23.6 |
| 0.2 | 86.3 | 80.5 | 68.4 | 65.2 | 63.5 | 41.1 |
| 0.3 | 87.0 | 86.9 | 83.3 | 78.9 | 77.1 | 55.7 |
| 0.4 | 91.4 | 89.1 | 87.7 | 84.0 | 83.9 | 70.7 |
| 0.5 | 94.9 | 91.5 | 90.9 | 91.4 | 86.3 | 78.0 |
Table 2: Effect of temperature on inhibition efficiencies of AEIB and NEIB on corrosion of Mild steel in H2SO4 environment.
Thermodynamic considerations
The temperature of the system was varied across the inhibitor concentrations from which the activation energy for the corrosion of Mild steel in solutions of H2SO4 was evaluated using the Arrhenius equation given in equation 3.
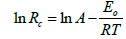 (3)
(3)
Rc is corrosion rate, Eo is apparent effective activation energy, R is general gas constant, T is temperature and A is Arrhenius preexponential factor [4]. Thermodynamic parameters: Enthalpy ΔH⃰i, Entropy ΔS ⃰i of AEIB and NEIB on Mild steel was calculated using (the transition state equation) expressed as shown in equation 4:
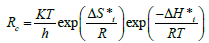 (4)
(4)
Calculated values of Activation energy between 313 and 333 K were obtained from the slope of Figures 2a and 2b and presented in Table 3. The values obtained for AEIB, NEIB are greater than those for the blank indicating the inhibition of Mild steel corrosion. Since the Activation energy which is the energy required to oxidize the metal, increased with inhibitor concentration, it implies that more energy has to be supplied to the system for the corrosion to take place thus, the observed decrease in corrosion rate. The values are also consistent with the data expected for the mechanism of physical adsorption (<80 KJmol-1) according to [11]. The reaction becomes more endothermic with increased inhibitor concentration. The positive values for ΔS*i (Table 3) show the spontaneous dissolution of the Mild steel sheet and and the increase in its value suggests decrease in disordering in the rate determining step. Values of enthalpy and entropy of adsorption are recorded in Table 3. It can be deduced from ΔH*i values that the adsorption of the inhibitor on Iron sheet surface is exothermic [12] (Figures 3a and 3b).
| AEIB | NEIB | |||||
|---|---|---|---|---|---|---|
| Conc. (g/L) | Ea | -∆H*i kJ/mol | ΔS*i kJ/mol | Ea | -∆H*I kJ/mol | ∆S*i kJ/mol |
| Blank | 28.4 | 25.8 | 30.4 | 28.4 | 25.8 | 30.4 |
| 0.1 | 56.8 | 54.2 | 51.2 | 54.6 | 52.0 | 49.2 |
| 0.2 | 62.2 | 59.6 | 64.5 | 49.6 | 47.0 | 30.2 |
| 0.3 | 38.6 | 36.0 | 13.9 | 58.4 | 55.8 | 54.8 |
| 0.4 | 42.9 | 40.3 | 2.7 | 52.7 | 50.1 | 33.8 |
| 0.5 | 52.2 | 49.6 | 23.9 | 66.3 | 63.7 | 74.2 |
Table 3: Thermodynamic parameters obtained from the adsorption of Ipomoea batatas leave extracts on Iron in acidic medium.
Adsorption consideration
The Temkin adsorption isotherm assumes that the measure of the surface coverage (θ) varies linearly with the logarithm of the inhibitor’s concentration (C) according to the following equation:
exp (-2θa)=C bads (5)
θ=-1/2a ln C-1/2a ln bads (6)
‘a’ is the molecular interaction parameter and bads is the stability constant of adsorption [6]. Equation 6 was obtained after simplifying and taking the logarithm of both sides of equation 5. A plot of θ against ln C will be linear with slope and intercept equal to 1/2a and -1/2aln bads respectively. Figures 4a and 4b present Temkin plots for the adsorption of the AEIB and NEIB on mild steel surface respectively. Table 4 revealed that Kads decreased with increasing temperature, which indicated that the plant extracts was adsorbed on the mild steel surface at lower temperature, however, the adsorbed extract desorbed at higher temperature; an indication of a physical adsorption mechanism [13].
| AEIB | NEIB | |||||||
|---|---|---|---|---|---|---|---|---|
| Temp (K) | Ln k | R2 | Slope | ∆Gads | Ln k | R2 | Slope | ∆Gads |
| 303 | 8.769 | 0.9581 | 0.117 | -32.21 | 5.146 | 0.935 | 0.199 | -23.08 |
| 313 | 8.133 | 0.992 | 0.123 | -31.61 | 3.354 | 0.968 | 0.341 | -19.18 |
| 323 | 4.502 | 0.9829 | 0.269 | -22.87 | 2.907 | 0.981 | 0.344 | -18.59 |
Table 4: Results from Temkin isotherm for adsorption of AEIB and NEIB on Mild steel in 1 M H2SO4.
Quantum chemical study
The computational study was done using the Vamp and the Dmol3 program which incorporates the Density functional theory (DFT) to investigate the interaction between the metal and the inhibitor. [9] reportedly isolated some chemical constituents from the leaves of purple sweet potato, which included Eugenol and Indole-3-aldehyde. Eugenol and Indole-3-aldehyde were picked from a random sampling of the many isolated compounds to represent the non-alkaloid and the alkaloid fractions of the leaves extract respectively. Table 5 shows the values obtained from DFT for the energy of the HOMO (EHOMO), the ELUMO and the ΔE. The EHOMO correlates with the electron donating capacity of the specie. A higher value of EHOMO is thus associated with better inhibitor. On the other hand ELUMO is connected with the affinity of the specie to accept electron [14]. Thus, the lower value of ELUMO implies better efficiency. The energy gap of a molecule ΔE is related with the hardness and softness of the specie. Larger energy gap implies that the molecule will need much energy to move from HOMO to the LUMO. Therefore, larger energy gap shows that the molecule is hard, while lower energy gap points toward soft molecule [15]. From the results presented in Table 5, it is evidence that indole-3-aldehyde is a better inhibitor than and eugenol. The highest occupied molecular orbital (HOMO) gives information about the regions in the molecule with the most energetic electrons, which are the electrons most likely to be donated to the electron poor species [16]. Table 6 shows the HOMO diagrams for the studied compounds. The results show that the HOMO is distributed across the studied compounds; as this goes to account for the inhibition abilities demonstrated by the AEIB and NEIB. The LUMO is the unoccupied orbital that has the lowest energy and gives information about the regions in a molecule that have the highest tendency to accept electrons from an electron rich species [17,18]. The diagrams reported in Table 6 show the LUMO diagrams for the studied compounds.
| Molecule | EHOMO (eV) | ELUMO (eV) | ΔE (eV) |
|---|---|---|---|
| Eugenol | -5.332 | -0.943 | -6.257 |
| Indole-3-Aldehyde | -5.569 | -2.283 | -7.852 |
Table 5: Results from Density Functional Theory (DFT).
| Compound | EUGENOL | INDOLE-3-ALDEHYDE |
| Optimized Structures | 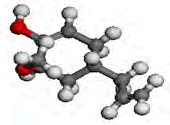 |
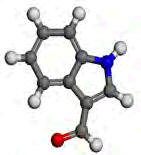 |
| HOMO | 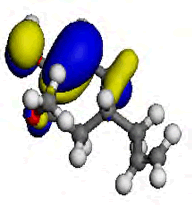 |
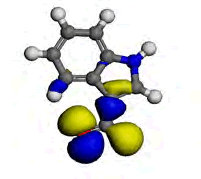 |
| LUMO | 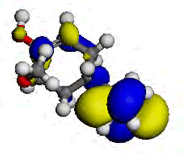 |
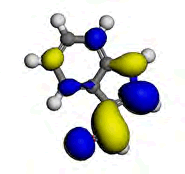 |
Table 6: The optimized geometric structures from Vamp, HOMO and LUMO diagrams from Dmol3.
AEIB, NEIB leaves act as good corrosion inhibitors for Mild steel in 1 M H2SO4 solution. The highest valuable result for corrosion inhibition performance was achieved at concentration of 0.5 g/L for AEIB and NEIB at 303K. The leave extracts have a very close inhibitory effects but AEIB is preferable to NEIB. The adsorption of extracts of inhibitors obeyed the Temkin isotherm. Physical adsorption mechanism has been proposed for the adsorption process following the results from thermodynamic parameters. Inhibition is due to the formation of the film on the metal/acid solution interface through adsorption of extracts of Ipomoea batatas leaves extracts on the metal surface. The results obtained from the computational study goes to back-up the experimentally obtained results.