Anatomy & Physiology: Current Research
Open Access
ISSN: 2161-0940
ISSN: 2161-0940
Research Article - (2015) Volume 5, Issue 2
Keywords: Knee; Pressure, Volume, Etiology, Nutrition, Stability, Flow
MCL: Medial Collateral Ligament; SMCL: Superficial Medial Collateral Ligament; DMCL: Deep Medial Collateral Ligament; MM: Medial Meniscus; FTPTA: Femoral-Tibial to Patellar Tendon Area; FTPTV: Femoral-Tibial to Patellar Tendon Volume; MRI: Magnetic Resonance Imaging; P: Pressure of FTPTV; V2: FTPTV (fatpad + synovial fluid); C: Constant
Research by Alexander et al. on live humans documented positive gauge pressures in the intra-articular knee joint space during terminal arcs of active extension and flexion, whereas between these two extremes, gauge pressures were negative [1]. Etiologies of these pressure variations have not been established.
Intra-articular knee joint space negative pressure etiology studies by others have considered micro-structure transfer processes such as plasma and lymph colloid osmotic pressures [2,3]. These microstructure processes have not been proven as being significant in diarthroidal joint negative pressure causation. Normal human knee motion causes intra-articular joint space pressure changes that subserve movement of fluid into and out of the intra-articular joint space [2,4].
Essential in knee pressure-volume causation is the articular space. The articular space has central and peripheral boundaries. Centrally, they are the proximal tibia and distal femur including its metaphysis. Peripherally, circumferentially disposed soft tissues (Supplementary Data, Item 1), which includes the patella with its horizontal retinacula and the quadriceps femoris tendon, all enclosed in deep fascia, constitute its external boundary. These tissues form a nearly closed sleeve. Within these boundaries the articular space includes the mobile amalgam of soft tissues, intra-articular knee joint space and popliteal space. The mobile amalgam is comprised of noncalcifiedchondral cartilage of the proximal tibia, distal femur and patella plus cruciate ligaments, popliteus tendon, menisci, synovium and associated fat. The intra-articular knee joint space, containing only synovial fluid, is bordered by chondral and meniscal cartilage plus synovium. The popliteal space contains neurovascular structures that service the knee, leg and foot.
This study aims to reveal how motion may cause supraatmospheric (positive) and subatmospheric (negative) pressures in the knee articular space. However, the analytical portion of this study (MRI) concentrates on FTPTV, a subset of the articular space, and projects the pressure/volume changes to similarly apply to the remainder of the articular space. It is intended to indicate how these pressures may support chondral nutrition, meniscal load bearing and joint stability as well as popliteal arterial and venous flow.
A normal right knee of a fit 55 year male (172 cm, 68.2 kg) subject was evaluated clinically, photographically, radiographically and per magnetic resonance imaging (MRI) qualitatively and quantitatively.
The subject had no history of pain, injury or compromise. He is regularly involved in Eastern mental/exercise programs and bicycling.
External topographic anatomy
Observation anteriorly and palpation medially of the right knee are performed on the subject at 0°, 35° and 130°.
Internal gross anatomy
Magnetic resonance imaging: MRI was performed on the subject using a 1.5 Tesla MRI unit with flex coil (Symphony Siemens; Erlangen, Germany). Sequences include a combination of proton density and T2-weighted imaging. Scans are performed every 0.4 cm at 0° and 35° in both sagittal and axial planes (Supplementary Data, Item 2, yz and xy planes respectively). Imaging is undertaken to determine change in volume of the infra-patellar fat pad in moving from 0° to 35°. Application of Siemens brand computer software allows freehand regions of interest (ROI) to be outlined on sagittal images, thus providing area quantification capability. ROI values were obtained by MRI dedicated radiologist and MRI dedicated radiology technician. A mechanical engineer derived formulas and performed all calculations.
During the 0° exam, a soft support is placed posterior to subject’s right heel to prevent support pressure on the calf, knee and distal thigh. During the 35° exam, the right heel is buttressed plantarward against a sand bag secured to the MRI table top, thereby maintaining the knee at 35°. The supine subject maintains slight quadriceps femoris tone during 0° and 35° exams in order to put the extensor hood in a functional state.
Considering any sagittal plane, the portion of the articular space that specifically defines the femoral-tibial to patellar tendon area (FTPTA) anteriorly is the patellar tendon mechanism plus deep fascia and posteriorly the: (1) anterior non load-bearing infra-patellar calcified femoral and tibialchondral surfaces; (2) anterior margins of the menisci; and (3) inter-condylar notch synovium. FTPTA, principally infra-patellar fat pad, includes its associated synovium and synovial fluid.
Radiograph: A lateral radiograph of subject’s weight-bearing right knee was taken at 130°. Radiograph was used at 130° instead of MRI because available MRI chamber space was insufficient to accommodate the 130° exam.
External topographic anatomy
Bulgings, as evidence of articular space positive pressure, occur about the knee during advanced arcs of extension and flexion. Most evident were bulging parapatellar tendon fat pads beneath skin and deep fascia. These bulgings were positioned between the patellar tendon and medial as well as lateral vertical patellar retinacula (Figure 1a and e). Less obvious visually, yet evident on palpation, is bulging of the medial joint line at 0° and 130° (Supplementary Data, Item 4a and c). In these advanced arcs, atmospheric pressure was less than articular space pressure [1]. The difference between these pressures and atmospheric pressure is mathematically expressed as differential pressure (ΔP):
Figure 1: Right knee topographical, MRI @ 0° and 35°, and X-ray @ 130°. (a) Frontal view photograph at 0°. (b) MRI sagittal image at 0°. (c) Frontalmedial oblique view photograph at 35°. (d) MRI sagittal image through femoral condyle at 35°. (e) Frontal view photograph at 130°. (f) Lateral view X-ray at 130°. 1 bulging medial and lateral parapatellar tendon fat pads; 2 depressed medial parapatellar tendon area; 3 FTPTA; 4 patellar tendon; 5 quadriceps femoris tendon.
Therefore
ΔP was negative in advanced extension and advanced flexion, indicating positive gauge pressure within the knee articular space [1].
Depressions about the knee, as evidence of articular space negative pressure, occur during midflexion. The most evident depressions are in the parapatellar tendon as well as the anterior medial (Figure 1c) and anterior lateral retro-patellar tendon plane regions. Less obvious visually, yet evident on palpation, is depression of the medial joint line at 35° (Supplementary Data, Item 4b). In midflexion, because atmospheric pressure is greater than articular space pressure, ΔP is positive, indicating negative gauge pressure within the knee articular space [1].
Internal gross anatomy
Magnetic resonance imaging: MRI axial images reveal the popliteal artery and vein enlarged when moving the knee from 0° to 35° indicating increased flow capacity at 35° (Figure 2b and d). At 0°, synovial fluid and fat pad venous engorgement are absent, however at 35°, gross synovial fluid and infra-patellar fat pad venous engorgement are discovered (Figure 2a and c). A single sagittal image is selected to illustrate the relative sizes of FTPTA and gross synovial fluid portions of FTPTA at 35° per ROI (Figure 2e and f).
Figure 2: Right knee MRI. (a) Sagittal view through medial aspect of lateral femoral condyle at 0°. (b) Axial view through femoral condyles at 0°. (c) Sagittal view through medial aspect of lateral femoral condyle at 35°. (d) Axial view through femoral condyles at 35°. (e) Sagittal view through inter-condylar notch at 35° with FTPTA ROI. (f) Sagittal view through inter-condylar notch at 35° with synovial fluid ROI. 1 popliteal vein; 2 popliteal artery; 3 stenosed popliteal vein; 4 narrowed popliteal artery, intraluminal diameter = 0.33 cm; 5 patellar fat pad, associated synovial fluid loculations; 6 grossly dilated patellar fat pad venous structures; 7 peri-anterior cruciate synovial fluid loculation; 8 engorged popliteal vein; 9 distended popliteal artery, intraluminal diameter = 0.45 cm; 10 ROI is FTPTA at 35°; 11 ROIs are gross synovial fluid portions of FTPTA at 35°.
Incrementally by one, sequentially paired lateral to medial FTPTA values are averaged and totaled. The first and last slice values are zero in that they do not intersect FTPTA. Total FTPTA is multiplied by 0.4 cm MRI slice thickness to approximate femoral-tibial to patellar tendon volume (FTPTV). FTPTV is comprised of the patellar tendon fat pad and its associated synovial fluid plus anterior infra-patellar, non load-bearing, noncalcified femoral and tibialchondral cartilage. Such noncalcified femoral and tibialchondral cartilage is indistinguishable from the underlying calcified cartilage plus condylar and intercondylar cortical bone when using a 1.5 Tesla MRI unit and flexcoil. Under this imaging method, the noncalcified cartilage portions of FTPTV cannot be quantified volumetrically. These cartilaginous bone coverings have relatively small volumes compared to the infra-patellar fat pad and also, volumes at 0° and 35° are estimated to be nearly equal. Consequently, their influence on FTPTV change from 0° to 35° is negligible and therefore are neglected in this study. Accordingly the patellar tendon fat pad plus its associated synovial fluid represent the overwhelming majority of FTPTV and are each evident on sagittal plane MRI (Figure 2a and c). The total area defined by their en bloc perimeter borders accurately depicts relative area change from 0° to 35° and is used for approximating FTPTV according to the following generalized equation:
where:
FTPTA= femoral-tibial to patellar tendon area in sagittal y-z plane
i=MRI sagittal slice number;
FTPTV= femoral-tibia to patellar tendon mechanism volume
FTPTV was approximated according to the following equation:
The percent change of FTPTV when moving from 0° to 35° was approximated as follows:
where:
(Figure 1b and d, Table 1).
Table 1: FTPTA & FTPTV @ 0° and 35° in sagittal plane per MRI.
FTPTA synovial fluid collections are discovered in the infrapatellar and inter-condylar notch spaces, however only in the 35° exam (Figure 2a through d).
The synovial fluid volume portion of FTPTV35 (FTPTVSF35) is obtained by averaging, incrementally by one, sequentially paired infra-patellar and intercondylar notch synovial fluid area values in the sagittal view. The first and last slice values are zero in that they do not intersect FTPTASF35. These values are totaled and multiplied by 0.4 cm slice thickness according to the following equation:
where:
Therefore, the relative percent change of FTPTV when moving from 0° to 35° represented by synovial fluid is approximated as follows:
(Figure 2e, f and Table 2).
Table 2: Synovial fluid in FTPTA & FTPTV @ 35° in sagittal plane per MRI. 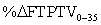 that is
that is 
Strength of this study is that it first reported MRI with extensor mechanism under tension demonstrating production of gross synovial fluid and enlargement of the popliteal artery and vein under negative pressure at 35°. It offers an explanation for the enigma of chondral cartilage nutrition, ΔP’s effect on meniscal load bearing, and joint stability. Finally, it offers an additional consideration regarding popliteal venous blood (knee, leg, and foot) transfer to the femoral vein. Weaknesses include the hypothetic nature of this single subject study.
Cyclical positive and negative intra-articular knee joint space pressures relate to joint angle during motion. The rate of change of intra-articular knee joint pressure is related to anatomic features of joint capsule with surrounding soft tissues plus those of the femur and tibia [4]. The relationship between joint angle and synovial fluid pressure is of considerable physiologic significance because the rate of fluid absorption from an intra-articular joint space varies with pressure [1,2].
Etiologies of pressure variations
Ventilation, the process of alternating movement of anabolic laden atmospheric gas into then catabolic laden gas out of the lungs, is a function of the thoracic cage creating pressure-volume variations. Conceptually, the process of providing nutrition for knee chondral cartilage similarly involves movement of anabolic laden fluids into then catabolic laden fluids out of the intra-articular joint space portion of the articular space. These fluid movements are generated by pressures resulting from potential volume changes with motion. Potential volume is the volume of the articular space or any portion thereof, at any arc position, if that space is at atmospheric pressure (Supplementary Data, Item 3 c and d, Cases 0 and 1).
Articular space is nearly closed. The exception is that the vascular system has ingress and egress. Since content volume is nearly fixed, pressure varying with motion means motion is associated with changes in articular space volume capacity. During advanced flexion or extension the volume of the contents within the articular space is greater than the articular space volume capacity at atmospheric pressure. Thereby, gauge pressure becomes positive. During mid-flexion the volume of the articular space contents is insufficient to fill the articular space volume capacity at atmospheric pressure resulting in negative gauge pressure (Supplementary Data, Item 3e, Case 2).
However, since the articular space is nearly closed means that it is partially open. As a result, both pressures and content volume adjust in response to motion. Negative gauge pressures of mid flexion enlarge FTPTV by causing fat pad venous and interstitial tissue engorgement plus increasing SF. Positive gauge pressures of advanced arcs decrease FTPTV by minimizing infrapatellar fat pad venous and interstitial fluid volumes as well as decreasing SF.
Condyles of the femur each have two distinct, nearly circular cams, one anterior and the other posterior. The central portion between these cams has a greater radius of curvature (flatter) than either the anterior or posterior cam [5]. The central flatter portions of the femoral condyles, which are load-bearing through the majority of the superior medial and superior lateral tibial articular surfaces (tibial plateaus) at 0°, are non load-bearing at 35°. Synchronously, when moving from 0° to 35° the posterior cams move from posterior and non load-bearing to mid-tibial plateaus and load-bearing which substantially decreases femoral-tibial contact areas and increases noncontact femoral-tibial space. The previously non-articulating anterior cams engage the patella, pushing it forward, thereby pulling its tendon and vertical retinacula anteriorly. This enlarges the potential space between the anterior patellar tendon mechanism plus deep fascia and the posterior: (1) non load-bearing infra-patellar calcified cartilage of the femur and tibia, (2) menisci and (3) inter-condylar notch synovium. The product of FTPTA and mediolateral boundaries of this anatomy defines FTPTV. At 35° the volume of the infra-patellar fat pad is less than the maximum potential FTPTV at atmospheric pressure, resulting in negative pressure. A plastic model demonstrates negative pressure production (Supplementary Data, Items 2 and 5).
When moving from 0° to 35°, as the inferior patellar-pole moves anteriorly, the extensor mechanism angulates about the superior patellar-pole quadriceps tendon and inferior patellar-pole patellartendon attachment sites. The patella being supported by the prominent anterior condylar cams is then positioned to separate the quadriceps femoris tendon from the distal femoral metaphysis. Consequently, the interposed suprapatellar pouch experiences decompression. A positive value for ΔP is associated with incurving of the quadriceps tendon.
This incurving is consistent with negative gauge pressure between the quadriceps femoris tendon and the distal femoral metaphysis (Figure 1d).
During advanced extension, the anterior femoral and patellar articular cams are dwelled which decreases potential FTPTV from that during midflexion. This occurs by bringing the patellar tendonretinacula structures closer to the anterior infra-patellar femoral-tibial non-load bearing calcified cartilage as well as menisci and inter-condylar notch synovium. Concomitantly, the hamstrings and gastrocnemius tendons move nearer to the posterior femoral-tibial condyles. Both events result in decreased noncontact femoral-tibial space. The posterior capsule-oblique popliteal ligament complex compresses the mobile soft tissues postero-centrally. Additionally, tough horizontal fibers of the popliteal fascia through their attachment to fibers of the fascia lata compress the posterior knee including the popliteal space. This occurs because fascia lata fibers that attach directly and indirectly to the proximal tibia anteriorly are taut at 0°. This spatial configuration causes compression of the articular space positioned between central rigid osseous structures and the peripheral circumferentially disposed musculotendinous, ligamentous and deep fascial structures. As a result, positive pressures develop in the articular space which includes structures within FTPTV as evidenced by outcurving of the patellar tendon (Figure 1b). Also, bulging occurs at the more elastic sites and especially where only deep fascia covers the mobile articular space fat. This is most evident in the medial and lateral parapatellar tendon regions (Figure 1a).
During advanced flexion, again the cams dwell, even though the anatomic configuration is different from that during advanced extension. In advanced flexion and extension, FTPTV is insufficient to contain the volume of patellar tendon fat pad plus synovial fluid at atmospheric pressure. This causes positive pressure within FTPTV structures, resulting in bulging of the parapatellar tendon regions during advanced flexion as during advanced extension (Figure 1e and a, respectively).
Gross synovial fluid and grossly dilated patellar tendon fat pad veins are discovered on MRI during midflexion, but not during advanced extension (Figure 2c and a, respectively). It is proposed that increase in FTPTV from advanced flexion and extension to midflexion is due to influx of plasma dialysate as synovial fluid and as infra-patellar fat pad venous and interstitial tissue engorgement. Efflux of these fluids occurs when pressures become positive during advanced flexion and extension.
An inverse relationship exists between soft tissue-synovial fluid pressure and FTPTV. Relatively high FTPTV during midflexion corresponds to negative gauge pressure and relatively low FTPTV during advanced extension corresponds to positive gauge pressure on a continuum basis during motion [1]. Assessment of FTPTV with MRI during advanced flexion of 135º was not obtained due to insufficient chamber space at 135º. However, bulging of positive pressure at 0º was similar to bulging at 135º.
where:
P = pressure of FTPTV
V2 = FTPTV (fat pad volume + synovial fluid volume)
C = constant
Effects of pressure variations
Proposed benefits of positive and negative pressures in the articular space include chondral cartilage nutrition, joint stability and vascular flow.
Chondral cartilage nutrition
The source of chondral cartilage nutrition has been characterized as an enigma. The very small chondral cartilage pores should permit only low molecular weight diffusion, not only into but out of this tissue. Diffusion is slow. Measured diffusion times range from 10 seconds to one hour. However, adequate nutrients somehow freely and rapidly migrate from sub-synovium capillaries to chrondocytes and back [6]. Most contend that diffusion from sub-synovium capillary bed to synovial fluid, then to and through chondral cartilage matrix and finally into chondrocytes, is primary if not solely chondral cartilage’s anabolic nutritional source pathway [6-9].
In the exercised rabbit knee, higher synovial fluid volumes than in the un-exercised knee were evident, which may have nutritional significance [10]. MRI studies of the subject reveals substantial volumes of gross synovial fluid at 35º whereas none is evident at 0º. It is proposed that increasing plasma dialysate synovial fluid volume with motion rapidly transports large amounts of concentrated anabolic nutrients en bloc into the intra-articular joint space, substantially circumventing diffusion and increasing synovial fluid nutrients over that during rest. The chondral cartilage dynamics of load-bearing compression-fluidexpulsion and non load-bearing decompression-fluid-absorption states (pumping) on intra- and extra-chondral cartilage fluid flow, to and from synovial fluid respectively, are significant for normal chondral cartilage nutrition [9-11].
Alternating positive and negative pressures measured within the intra-articular joint space synovial fluid are not limited to the intraarticular joint space per se. These pressures exist throughout the articular space, including the popliteal space. The subject’s luminal cross-sectional area of the popliteal artery increased by 86% when it experienced negative pressure at 35º as compared to positive pressure at 0º (Figure 2d and b, respectively). Under pulse pressure, this would substantially increase flow.
Experiencing negative pressure, sub-synovium arterioles and capillaries dilate, decreasing arteriole and capillary bed peripheral resistance, which increases blood flow to and through the capillary bed. In rabbits, synovium is characterized as a sheet of non-epithelial cells separated by interstitium filled gaps beneath which are fenestrated capillaries. These fenestrations act as filters and are directed toward the synovium [12,13]. Therefore, it follows that during capillary dilatation; fenestrae enlarge, thereby providing increased porosity. This together with increased capillary flow, allows increased plasma dialysate transfer from capillaries to the intra-articular joint space via the synovium extracellular matrix. Synchronously, during negative pressure, the popliteal artery enlarges providing increased arterial blood supply capacity. Consequently, joint nutrition may be enhanced by increasing anabolite loaded synovial fluid influx (plasma dialysate) mixing with catabolite loaded synovial fluid. Results on the subject reveals movement from 0º to 35º increases FTPTV by 6.3 cm3 (22.6%) of which 1.9 cm3 (30.2%) is synovial fluid (Figure 2a, e and f, Tables 1 and 2).
Intra-articular joint space aspiration during negative pressure enhances synovial fluid influx. The gaps between synovial cells contain a complex interstitial matrix comprised of several types of collagen fibrils and glucosaminoglycans, creating hydraulic resistance [12]. The extra-synovium articular space soft tissues also experience interstitial hydraulic resistance. During midflexion, the mobile amalgam of soft tissues is subjected to negative pressure. Fluid transfer under this condition experiences different flow rates dependent on fluid viscosity and interstitial tissue resistance. Because it lacks interstitium resistance, the relatively large potential space of the knee intra-articular joint space provides a reservoir of minimal intrinsic flow resistance during the sub-atmospheric pressure phase. The subsynovium capillary bed is approximately 4-11 μm from the intra-articular joint space [12]. Synovium does not have a basement membrane, making it more porous than if it otherwise had a basement membrane [14,15]. It then follows that the intra-articular joint space experiences a modicum of aspiration during midflexion because of having less resistance to flow than the surrounding soft tissues, proximity to the subsynovium capillary bed and positive ΔP.
Intra-articular joint space motion induced positive and negative pressures are major determinants of fluid exchange across synovium [4,16]. The filtration process is facilitated by negative pressures of midflexion; it follows that the absorption phase occurs during positive pressures of advanced flexion and advanced extension.
Catabolite removal from sub-synoviuminterstitium is effected by both hematic and lymphatic capillary systems. Regarding the hematic system, one explanation for removing sub-synovium interstitial fluid catabolites is that plasma depleted hyper-osmotic capillary blood, after having had the plasma dialysate (synovial fluid) forcibly removed by hydrokinetic active transport, imbibes the catabolite enhanced interstitial fluid into the hematic capillary bed by osmosis and diffusion. The phagocytic activity of the lymphatic capillary bed removes particulate matter including proteoglycan fragments, colloids and synovium detritus from the synovial fluid. The hyperosmolarity of lymph, resulting from particle ingestion into lymphatic capillaries, absorbs catabolite loaded interstitial fluid by osmosis and diffusion. A unique lymphatic one-way valve system associated with alternating pressures (positive and negative) moves lymph away from synovium and toward major lymphatic vessels. Electron-microscopic studies revealed that lymphatic capillaries have valvular structures to their tips. These endothelial cells overlap, causing their edges to flap back and forth, thereby acting as a valve [3]. Alternate compression and expansion of the synovial lymphatic plexus by alternating pressures of joint motion assists in catabolic fluid flow through the lymphatics [17].
Chondral load-bearing alternating with non load-bearing is a determinant of fluid movement out of and into chondral tissue, respectively. Chondral interstitial efflux is due to load-bearing compression deformation. As chondral cartilage unloads, it expands from its previously compressed state, which is associated with anabolite rich synovial fluid influx from intra-articular joint space into the chondral cartilage [9,11,18,19] (Supplementary Data, Item 6). Conceptually, these events provide sufficient nonvascular chondral cartilage nutrition during periods of high demand.
When the articular space pressure is positive at 0°, the three anterior and two posterior cams are non load-bearing and contiguous with a thin film of synovial fluid backed by synovium (Figure 1b). When the articular space pressure is negative during midflexion, these cams are load-bearing and mostly not contiguous with synovial fluid backed by synovium (Figure 1d). During the negative pressure phase of loadbearing motion, cam chondral interstitial catabolite loaded fluid is forced into the intra-articular joint space at and near load-bearing sites. This is evidenced by synovial fluid accumulations, especially about the patella-femoral articulation (Figure 2c). Simultaneously, hydrokinetic active transport influx of anabolite loaded plasma dialysate into the intra-articular joint space occurs which promotes mixing of their respective metabolites. These fluid transfer processes associated with midflexion reverse during arcs of advanced extension and advanced flexion. During these advanced states, articular space pressures are positive and all three cams are mostly non load-bearing, and therefore contiguous with synovial fluid backed by synovium. This allows chondral interstitial fluid reconstitution by three mechanisms. First, the electrical disparity between negatively charged compressed chondral cartilage aggregans and positively charged interstitial and synovial fluid counterions such as calcium (Ca++) and sodium (Na+) force synovial fluid to flow into chondral interstitial spaces via osmosis obtaining overall electroneutrality as chondral cartilage decompression occurs. Second, mechanical memory during elastic compression deformation of chondral collagen and large proteoglycan fibrils during load-bearing assists reconfiguring chondral tissues to their non compressed state as load-bearing diminishes. This forcibly causes the expanding chondral cartilage to imbibe synovial fluid [9,11,19]. Third, positive intraarticular joint space pressure facilitates synovial fluid movement into expanding non load-bearing cartilaginous tissues as well as through synovium to the sub-synoviumperi-capillary interstitial spaces.
During motion, non load-bearing and load-bearing states are involved in fluid transfer from sub-synovium micro-vasculature to chondral cartilage and back, thereby providing chondral cartilage nutrition via active fluid transport without direct vascular supply.
Joint stability
Menisci bear load, provide knee joint stability and shock absorption while evenly dispersing synovial fluid [20].
The deep medial collateral ligament (DMCL) attaches to the periphery of the middle one-third of the medial meniscus (MM). The descending distal oblique ligament (posterior oblique ligament) and the ascending distal oblique ligament fibers of the superficial medial collateral ligament (SMCL) attach to the posterior one-third of the MM. The semimembranosus tendon partially inserts directly into and indirectly through the descending distal oblique ligament into the posterior MM [21-24] (Supplementary Data, Items 1 and 2).
In extension, the MM is partially forced medially out of the space between the femoral and tibial condyles, pushing and tensing the DMCL, providing meniscal load-bearing and stability. This is evident by the palpable bulging of the anterior two-thirds of the MM and MCL at full extension. However, when the knee moves from full extension to midflexion, the previously bulging MM and associated DMCL become palpably recessed due to net forces related to positive ΔP. One explanation is positive ΔP is associated with increased non loadbearing femoral tibial space during midflexion. ΔP would force the MM laterally into the interstices created by the femoral condyles changing from large to small load-bearing areas on the medial tibial plateau, creating more noncontact femoral-tibial space. This would cause loadbearing through the anterior two-thirds of the MM, providing stability by increasing load-bearing area and maintaining tension in the DMCL. When the knee moves from midflexion to advanced flexion the MM is forced medially, maintaining tension in the DMCL (Supplementary Data, Item 3).
As the knee actively moves from advanced flexion to midflexion pressures in the articular space change from positive to negative. At this time, the medial posterior femoral condyle translates anteriorly and rotates superiorly away from the posterior medial tibial condyle. These simultaneous events enlarge the space between the posterior medial femoral and tibial condyles (Figures 1f and d, respectively). Accordingly, net forces related to positive ΔP push the posterior MM inward to fill the impending femoral-tibial void, resulting in meniscal load bearing which provides stability.
Lateral meniscus is somewhat different than medial meniscus in form and function. However, from the perspective of pressure-volume, they are similar.
Vascular flow
The popliteal space’s diamond-shaped perimeter borders are the hamstrings (semimembranosus and semitendinosus) proximomedially and the biceps femorisproximo-laterally. Distally, the diverging gastrocnemius musculotendon heads complete the perimeter borders. The floor consists of the planumpopliteumfemorale proximally, fascia of the popliteus muscle distally and the posterior capsule-oblique popliteal ligament complex in the middle (Supplementary Data, Item 7). The roof is comprised of the strong mostly transverse fibers of the popliteal fascia, which attach to and are continuous with the fascia lata. This space contains major neuro-vascular structures; the most significant in this study are the popliteal artery and vein [24].
The popliteal space is unique in that during the negative pressure phase, an additional separate dynamic from that of the extensor mechanism and femoral cams cause this space to experience negative pressure. The additional cause of negative pressure is a function of hamstrings and gastrocnemius tendons-popliteal fascia mechanics (Figure 3).
Figure 3: Illustration of right knee medial views fascia lata and popliteal fascia. (a) At 0°, compression of popliteal space by popliteal fascia. (b) At 35°, medial popliteal fascia is pushed posteriorly, dynamized by bowstringing hamstring and gastrocnemius tendons decompressing popliteal space. 1 vertical fibers of fascia lata; 2 horizontal fibers of popliteal fascia; 2x popliteal fascia fibers wrapping 180° around medial hamstring tendons becoming the roof of popliteal space; 2y popliteal fascia fibers wrapping 180° around medial gastrocnemius musculotendon becoming the roof of popliteal space; 3 crural fascia fibers; 4 popliteal space; 5 gastrocnemius medial musculo-tendon; 6 medial hamstring tendons; 7 outcurving patellar tendon; 7Ꞌ incurving patellar tendon; 8 outcurving quadriceps tendon; 8Ꞌ incurving quadriceps tendon.
During extension, the popliteal fascia is taut from its anterior attachments to the extensor mechanism and proximal tibia via the fascia lata, thereby creating compression posteriorly. As a result of this compression, positive pressure develops in the popliteal space which compresses the popliteal vein, producing stenosis (Figures 2b and 4a). This explains why an individual, such as a soldier, standing at attention with knees locked in full extension, may experience venous pooling in knees, legs and feet sufficient to decrease circulating volume, contributing to syncope.
When the knee is flexed to near 35°, the popliteal fascia is pushed posteriorly on the medial and lateral sides, dynamized principally by bowstringing hamstring tendons and secondarily by gastrocnemius tendons, creating the centrally recessed popliteal fossa. Accordingly, this produces tension in the popliteal fascia that leads to and forms the dome of the popliteal fossa (roof of the popliteal space) [24]. Simultaneously, the posterior femoral condyles, which are prominent posteriorly at 0°, vacate their posterior positions by translating anteriorly to 35°. These events facilitate decompression by increasing potential space and therefore negative pressure in the popliteal space, causing popliteal vein dilatation and blood aspiration from the knee, leg and foot. This occurs because closures of femoral vein one-way valves prevent backflow (Figure 4b). When moving from 35° to advanced arcs positive pressures result, causing the engorged popliteal vein column of blood to be compressed, ejecting its contents through the adductor magnus muscle tendon hiatus into the femoral vein. This is facilitated by closure of calf and popliteal vein one-way valves (Figure 4a). As a result, catabolic nutrition of the knee, leg and foot is served. Such venous flow assistance explains why prolonged sitting should be avoided, as it promotes stasis which impedes coagulation factors from being diluted by flushing which may predispose to deep vein thrombosis and pulmonary embolism.
Alternating gross anatomic configurations of the moving knee, while performing as a bellows, causes pressure changes which physiologically facilitate chondral cartilage nutrition, knee joint stability and vascular flow. Rhythmical pressure variations in the articular space are a function of alternating engagement and disengagement of femoral condylar and patellar cams while being encased in a tough circumferentially disposed soft tissue sleeve. These pressure variations have an inverse relationship with FTPTV. Relatively low articular space pressure during mid-flexion corresponds to relatively high FTPTV, whereas relatively high articular space pressure during advanced extension and advanced flexion corresponds to relatively low FTPTV on a continuum basis during motion. PV2 = C.
During the negative pressure phase of mid-flexion, capillary plasma as a dialysate and load-bearing chondral interstitial fluid are forced into the intra-articular joint space. These conditions reverse during the positive gauge pressure phases of advanced flexion and advanced extension. Under all these conditions, nutrition is enhanced by metabolite exchange.
This study focuses on the normal human knee. Similar anatomic features of the human knee exist in other mammalian, reptilian and avian synovial joints and therefore similar pressure changes and their effects may exist in these joints.
It is recognized that this treatise contains several theoretical ideas. It is hoped and believed that corroborative evidence offered will promote curiosity and further investigation.
I wish to thank the following individuals for their specific work: William O. Irvine BSME BSCE MBA, engineering consultation and editing; ZacharieBrahmi PhD and Philip R. Reid MD, manuscript foreign language translation; Trena Lowery, library science; Eric Straily EIT, engineering computer graphics; Thomas Weinzerl MS and Chris Brown MS, medical illustration computer graphics; Timothy Yates MS, medical photography; Linda Mutchman BA, videography; Danny Cook BA and Karl Stoicheff, video editing; Thomas Vehey MD and Scott Etienne RT, MRI and ROI; Jay Donaldson, Don Altop, Todd Messick, and Hoagland Elliott, plastic model fabrication; ZacharieBrahmi PhD and Jack Farr MD, technical manuscript review; Min Li MD, assisted with photograph, x-ray and MRI presentations; and Kathy Flint RN MSN, editing, manuscript preparation and submission.
Conflict of Interest Statement
The author confirms that there are no conflicts of interest and no financial support was received for this work.
Funding
There are no outside funding sources.
Ethical Approval
The single subject under this study gave informed consent to the work and the study was approved by the Indiana Orthopaedic Hospital Institutional Review Board.