
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 4
Post-Translational Modifications (PTMs) encompass alterations to the side chains of amino acids subsequent to their synthesis. These modifications play pivotal roles in regulating diverse cellular processes, such as kinase activation, protein degradation and more. Additionally, they exert a significant influence on the functionality and architecture of the proteins within which these processes occur. Depending on the specific amino acid affected and its location, these modifications can contribute to the development of various diseases. Through Density Functional Theory (DFT) calculations, we delve into the structural ramifications of PTMs on amino acids, exploring whether they might contribute to their deregulation. We recognize that these modifications do indeed alter the orientation of amino acids, which may play a significant role in the development of various diseases or even their initiation. Additionally, we observe PTMs also have an impact on several characteristics of amino acids, such as dipolar moment, Highest Occupied Molecular Orbital (HOMO), Lowest Unoccupied Molecular Orbital (LUMO) and partial charge. This suggests that modifying these features can potentially influence factors like the stability of the amino acid or even the entire protein. Through this study, we can conclude that by adopting this approach, we can gain deeper insights into the influence of PTMs on amino acids and whether such influences can be directly linked to the development of diseases associated with PTM deregulation.
Arginine; Methylation; Serine; Phosphorylation; Dysfunction, Conformational analysis; Cancer; Semi empirical
Post-Translational Modifications (PTMs) are alterations to amino acid side chains after synthesis, significant for regulating cellular processes, such as kinase activation and protein degradation. Moreover, they exert a significant influence on the functionality and structure of the proteins within which these processes take place. Variations in these modifications can lead to the onset of various diseases, contingent upon which amino acid is affected and its specific location [1].
Of particular significance among PTMs is methylation, as it governs essential functions such as Deoxyribonucleic Acid (DNA) repair, protein-protein interactions and cell signaling [2].
Methylation of arginine, catalyzed by Protein Arginine Methyltransferases (PRMTs), is essential for cellular functions such as DNA repair and signaling. PRMTs transfer a methyl group from S-Adenosylmethionine (SAM) to the guanidino nitrogen atoms of arginine, producing three types of methylarginine: Two monomethylated and one dimethylated as shown in Figure 1 [2- 4].
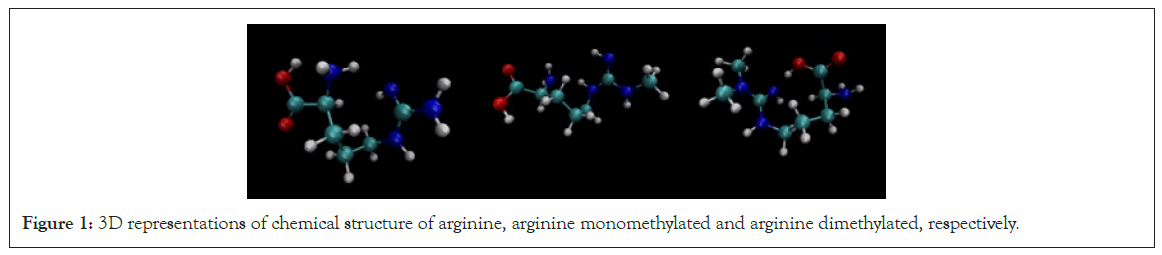
Figure 1: 3D representations of chemical structure of arginine, arginine monomethylated and arginine dimethylated, respectively.
The process of arginine demethylation remains a subject of ongoing investigation, as conclusive evidence for the existence of a dedicated enzyme with the exclusive capability to demethylate arginine is still lacking. However, a category of enzymes possessing this activity does exist alongside other functions. The group of Jumonji C (JMJD) domain containing proteins, renowned for activities such as tyrosine kinase activation, lysine hydroxylation and lysine methylation, has demonstrated its involvement in the regulation of arginine methylation acting as arginine demethylases [5].
Mutations or abnormal expression of PRMTs can deregulate arginine methylation, leading to diseases, such as cancer and neurodegenerative disorders. Arginine methylation plays a key role in gene expression and signal transduction and its dysfunction can result in aberrant cell growth, impacting cancer progression.
Arginine methylation also has an influence in Ribonucleic Acid (RNA) processing events like splicing and mRNA stability. And aberrant mRNA formation caused by arginine methylation can produce dysfunctional proteins and help in tumor development too.
Another important aspect affected is the response of the cell to DNA damage, cause the arginine methylation has an especially significant role in maintaining chromatin integrity preventing cancer development. So, the disruption of these processes is the key to cancer initiation and progression [6,7].
As it was said before, the loss of normal function of the arginine methylation can also help in neurodegenerative diseases development, like Alzheimer’s disease, Parkinson disease or even Amyotrophic Lateral Sclerosis (ALS).
One of the hallmarks of Alzheimer’s disease is related to Tau protein. This protein when hyperphosphorylated it aggregates disrupting the normal structure and function of the neurons leading to cellular death. Wrong arginine methylation of the Tau protein can change its phosphorylated status which can help it to aggregate and consequently the progression of the disease [8].
Another example is in the Parkinson disease, where something similar happens but with different protein. Alfasynuclein misfolding and aggregation cause loss of dopaminergic neurons which are related to the symptoms of Parkinson disease. Disruption of normal function of arginine methylation has been linked with the formation of the Lewis bodies, aggregates of alfasynuclein protein.
It is important to note that these correlations are still been studied and can vary depending on the protein involved. However, there is no doubt that deregulation of arginine methylation is a significant factor to cancer development.
The arginine dimethylated, although it can vary in some function it can affect the same processes and prejudice the normal cell function with its dysfunction [9].
Other PTMs that has been studied is the phosphorylation due to is large amounts of extremely important functions on our organism. Many enzymes and receptors are regulated by phosphorylation, one well known example is the tyrosine or serine kinase receptors, that are used as bridge in signal transduction on gene expression regulation.
Serine is one of the amino acids that suffer this modification, a reversible mechanism that occurs through protein kinases where a phosphate group (PO4) is added to the hydroxyl group on side chain of the serine residue from a molecule of adenosine triphosphate, as shown in Figure 2 [10,11].
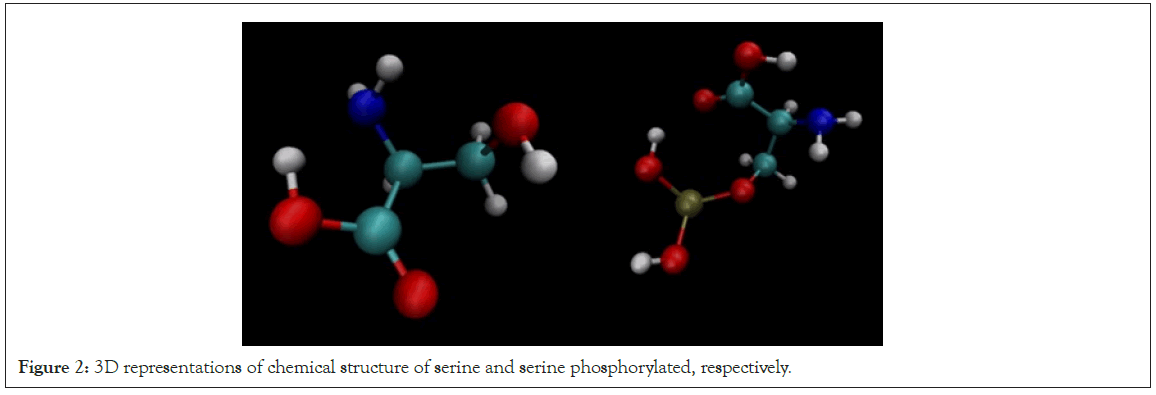
Figure 2: 3D representations of chemical structure of serine and serine phosphorylated, respectively.
Since it is a reversible modification, the phosphate group can be removed and in the case of serine is done by enzymes called serine phosphatases.
This process can be deregulated in many ways which can lead to cancer or neurodegenerative diseases development. Errors like overexpression or mutation of kinases or phosphatases and protein misfolding are examples that can deregulate the normal function of serine phosphorylation and cause different diseases. The impact of this deregulation, causing a dysfunction can vary in cancer for example, it may contribute to the development of the disease, due to its impact in cellular signaling leading too uncontrolled growth, survival and metastasis of the cell [12].
Like arginine methylation, it can activate oncogenes and inactivate tumor suppressor genes. Besides that, it can inhibit apoptosis by disrupting the balance between survival and death signal, which will help on cancer survival, all due to his role on signal transduction between extracellular and intracellular ambient, through serine/threonine receptors where after an external stimulus enzyme like protein kinase C will be activated and phosphorylate for example a protein involved on transcription regulation.
Certain proteins play a key role in cell migration and their activation hinges on the phosphorylation of serine residues. This phosphorylation facilitates cell motility, a process significant for various physiological functions. However, the disruption of this process can lead to an unrestrained escalation in the activity of cell invasive traits. Such dysfunctionality contributes to phenomena like cancer metastasis, where tumors gain the ability to readily infiltrate other organ tissues. This metastatic behavior significantly complicates treatment approaches, as the cancer spreads throughout the body, evading effective interventions.
Just like arginine methylation, the serine phosphorylation when deregulated can be related to neurodegenerative disorders development. The deregulation of serine phosphorylation has been implicated in pathogenesis of several diseases. In Alzheimer’s disease, it has a more direct implication, because Tau protein, when hyperphosphorylated, creates neurofibrillary aggregates, that are related to the disease development and this hyperphosphorylated state can be caused by the serine phosphorylation deregulation [13].
It can also be associated with Huntington’s disease, characterized as an abnormal expansion of Cytosine-Adenine-Guanine (CAG) repeats in the Huntingtin (HTT) gene, resulting in mutant proteins. Serine phosphorylation of mutant huntingtin can alter its conformation, aggregation and toxicity, contributing to the selective degeneration of neurons in the striatum and cerebral cortex, so its deregulation has been implicated in Huntington’s disease pathogenesis [14].
It is significant to acknowledge that these diseases have a multifactorial nature, implying that each individual case may not be attributed to the same underlying cause. Despite the evident correlation between these modifications and the aforementioned diseases, it's important to recognize that merely gaining a deeper understanding of the molecular function of these modifications may not directly lead to a solution for treating these diseases.
Conformer determination
The study included five groups: Unmodified arginine, methylated arginine, dimethylated arginine, unmodified serine and phosphorylated serine. Each group was analyzed in vacuum and aqueous environments.
We generated 30 conformers for each group (unmodified arginine, methylated arginine, dimethylated arginine, phosphorylated serine) in vacuum and water using Open Babel GUI, selecting the five conformers with the lowest formation heat for further analysis.
Lowest energy geometry determination
We selected the five lowest energy conformers from each group (in vacuum and water) and proceeded with Density Functional Theory (DFT) calculations. Input files for these calculations were generated using MacMolPlt (version 7.7.2). We use WB97X-D functional and 6-31G basis set. The theoretical studies were carried out with the aid of the GAMESS program.
Density functional theory calculation
Density Functional Theory (DFT) is a computational method used to study the electronic structure and properties of molecules by focusing on electron density rather than individual electron behavior.
This study employed WB97X-D functional and the 6-31G basis set to optimize molecular geometries.
The electron density represents the probability distribution of finding an electron in a particular position of space and contains all the information needed to describe the system's electronic properties.
It uses the Hohenberg-Kohn theorems as the foundation of its principles and Kohn-Sham equations to essentially simplify the many electrons problems, that happen for example in Schrodinger equations.
The exchange correlation functional is used to limit the exchange and correlation effects among electrons, but because the exact form of functional is generally unknown we tried to use one that is more accurate as possible depending on the molecule we are studying.
The basis set is also important since it is a set of functions that represent the electron wave functions within the system.
Visualization
From every resulting text file, from DFT calculations, we convert the more stable conformation in XMOL file for visualization and further analysis and all the 3D visualization was done on Visual Molecular Dynamics (VMD) including the image shown during this article.
Overlap of the conformers
The overlap of the conformers for conformational analysis was done in VMD by Root Mean Square Deviation (RMSD) method, where we choose 4 non-coplanar atoms to reach the orientation that best superimpose both molecules.
For the conformational analysis we compared some aspects, between conformations with the PTM and without PTM and between conformation in a solvent (water) and in vacuum, that we could get with DFT calculations.
The aspects we analyze were, the lowest energy of geometry in equilibrium, dipolar moment, Lowest Unoccupied Molecular Orbital (LUMO), Highest Occupied Molecular Orbital (HOMO) and partial charge of the atom that suffers the PTM on amino acid.
On Table 1 we can see the parameters obtained by DFT calculations, on the conformation with the lowest energy of geometry in equilibrium, in other words the more stable conformation, that helps reach some conclusions.
| Amino acid | The lowest-energy structure (kcal/mol) | Dipolar moment (Debye) | Lowest unoccupied molecular orbital (Hartree) | Highest occupied molecular orbital (Hartree) | Partial charge |
|---|---|---|---|---|---|
| Arginine vacuum | -3,80,36,85,381 | 76,52,891 | -0.3092 | 0.0857 | -0.790507 |
| Arginine water | -38,03,68,451 | 64,15,356 | -0.3072 | 0.0949 | -0.782663 |
| Arginine methylated vacuum | -4,05,02,25,286 | 87,70,407 | -0,2992 | 0.0932 | -0.675325 |
| Arginine methylated water | -4,05,00,88,847 | 57,01,412 | -0.2803 | 0.0574 | -0.676489 |
| Arginine dimethylated vacuum | -4,29,67,00,165 | 74,63,576 | -0.3139 | 0.0671 | -0.569853 |
| Arginine dimethylated water | -4,29,66,45,631 | 57,15,495 | -0.2667 | 0.0342 | -0.598985 |
| Serine vacuum | -2,50,18,47,022 | 47,74,451 | -0.3414 | 0.0675 | -0.588594 |
| Serine water | -2,50,18,21,354 | 58,27,341 | -0.3256 | 0.081 | -0.596117 |
| Serine phosphorylated vacuum | -60,62,42,526 | 69,89,128 | -0.3475 | 0.023 | -0.669108 |
| Serine phosphorylated water | -6,06,23,64,187 | 6,89,413 | -0.3425 | 0.0249 | -0.651164 |
Table 1: Analysed components of most stable conformation of each amino acid.
Arginine
When analyzing the dipolar moment of arginine, it becomes evident that its value is lower when it is in a solvent like water compared to when it is in a vacuum. This phenomenon, however, is not observed in the case of serine. This contrast indicates that the interactions between arginine and water play a significant role in influencing its dipolar moment. This influence is rooted in the relationship between the electronegativity of its constituent atoms and the lengths of its bonds.
When we extend our investigation to the differences in dipolar moments between various amino acids with and without modifications, arriving at a definitive conclusion becomes challenging. Notably, in a vacuum, the dipolar moment of methylated arginine surpasses that of unmodified arginine, whereas dimethylated arginine exhibits nearly identical dipolar moment as unmodified arginine. In an aqueous environment, methylation and demethylation of arginine lead to a reduction in its dipolar moment. However, the distinction between these two dipolar moments remains relatively modest. the amino acids without and with the modifications.
Analyzing the difference between HOMO and LUMO on vacuum, there is no notable difference between arginine with no modification and arginine with modifications. But when we change the solvent to water, we note a big difference, since the difference is lower each modification which may indicate that each methylation can alter the stability of the amino acid or even the protein, since it can make then more vulnerable to interactions with other components.
The partial charge is created by the asymmetric distribution of the electrons in a chemical bond and when we look at partial charge of the atom that suffers the PTM, shown in Figure 3 the arginine we realize that each modification decreases his partial charge which makes sense since the electrons are used to create a new chemical bond.
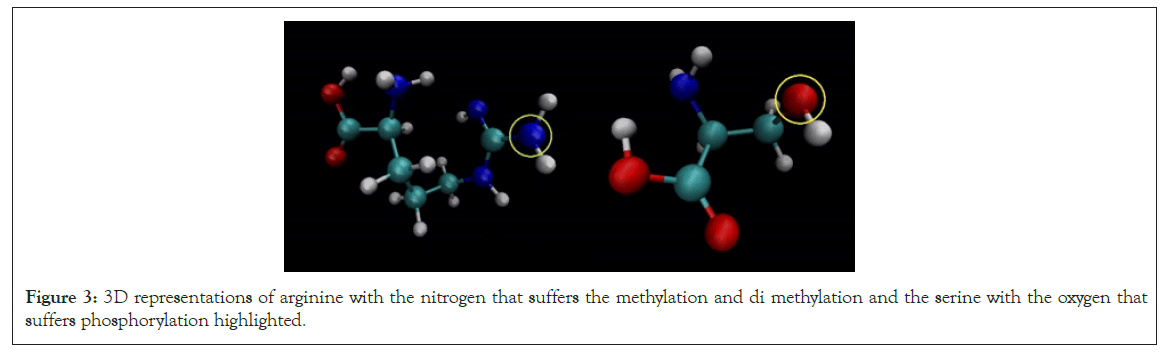
Figure 3: 3D representations of arginine with the nitrogen that suffers the methylation and di methylation and the serine with the oxygen that suffers phosphorylation highlighted.
Looking at the structures of the amino acids and comparing before and after the PTMs we can say that the modification can alter the structure and orientation of the amino acid which may directly or indirectly affect its stability.
As is shown in Figure 4, the methylation not only changes the orientation of the side chain but also of the amino group and the carboxylic acid. This can be noted in both ambient, vacuum and water.
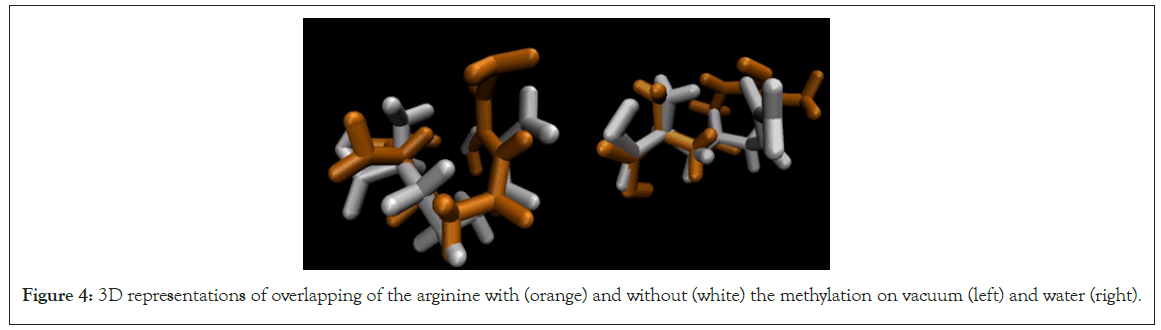
Figure 4: 3D representations of overlapping of the arginine with (orange) and without (white) the methylation on vacuum (left) and water (right).
When we examine the comparison between arginine with di methylation and its unmodified counterpart, shown in Figure 5, a significant impact becomes apparent, particularly in relation to its side chain. This is evident through a substantial alteration in the orientation of the side chain both in vacuum and in a water environment.
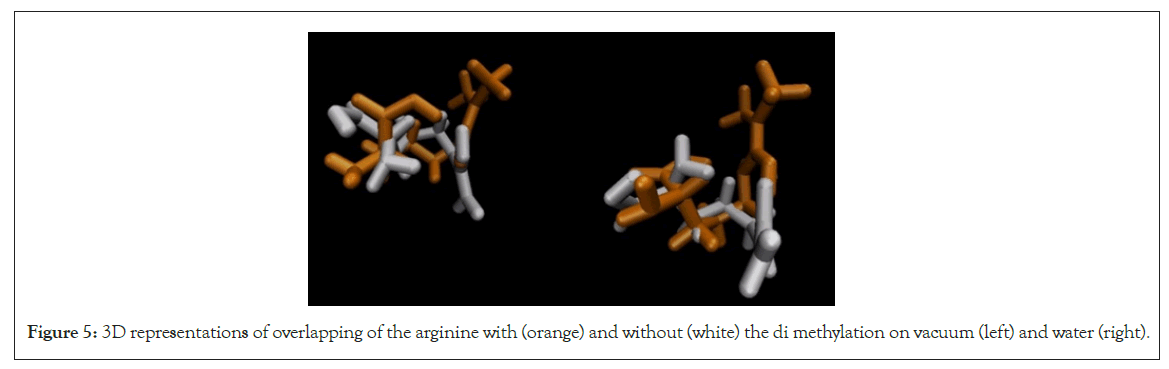
Figure 5: 3D representations of overlapping of the arginine with (orange) and without (white) the di methylation on vacuum (left) and water (right).
Serine
Upon analyzing serine and its phosphorylation, notable distinctions emerge when compared to the effects of arginine methylation. When we examine the dipolar moment, it becomes evident that this modification leads to an increase in both solvents.
When we observe the disparity between the HOMO and the LUMO in serine, a more pronounced decrease is evident compared to methylation in both solvents. This observation implies that phosphorylation renders the amino acid even more unstable, potentially contributing to the disruption of processes involving it. This instability could be a contributing factor to the onset of various diseases, as mentioned earlier.
The results of phosphorylation stem from a reaction involving amino acid and a group composed of a phosphorus atom and three oxygen atoms. Given the substantial size of this group, it likely exerts an electron pushing effect towards the oxygen atoms it is bonded with. This phenomenon could elucidate the rationale behind the augmentation in the partial charge of the oxygen atom linked to the phosphorylated site.
Analyzing the serine, the difference is not that notorious, but we still can see in Figure 6, especially in water some influence of the phosphorylation on the structure of the amino acid.
This study demonstrates that the deregulation of arginine methylation and serine phosphorylation is not haphazard and more significantly, it elucidates the molecular implications of these PTMs for amino acids. These insights are significant in comprehending the mechanism behind their deregulation, ultimately contributing to the onset of various diseases.
While further investigations are necessary to yield more robust results, this study unequivocally confirms the influence of PTMs on both the structural integrity of amino acids and the proteins within which they reside. Beyond the observable disparities depicted in the figures generated by this study, other computational analyses employing DFT also reveal alterations induced by PTM influence.
In conclusion, the meticulous analysis of these types of modifications through conformational analysis offers a pathway to gain deeper insight into their potential deregulation. This, in turn, paves the way for devising strategies to prevent the emergence of diseases stemming from the disruption of normal PTM functionality, which is indispensable for maintaining the optimal functioning of the human organism.
The datasets generated and analyzed during this study are publicly available on GitHub at (https://github.com/martims22/ Conformational-analysis-of-arginine-and-serine-in-silico) (https:// github.com/martims22/Conformational-analysis-of-arginineand- serine-in-silico) under an open-access license.
I would like to extend my sincere gratitude, firstly, to my advisors, Dr. Paulo Abreu and Dr. Jorge Marques. Their profound knowledge and invaluable experience have been instrumental in shaping my academic journey. I am particularly appreciative of the time they dedicated to me, as they consistently exhibited unwavering availability to offer their assistance in various capacities.
In addition to my advisors, I wish to express my heartfelt thanks to the University of Coimbra, specifically the Faculdade de Ciências e Tecnologia, for providing me with the necessary resources to conduct my experimental work. Their support has been pivotal in the successful execution of my research endeavours.
Lastly, I would like to extend a special thank you to Professor Dr. Luís Arnaut. His gracious offer of an internship within the chemistry department of the University of Coimbra has afforded me the remarkable opportunity to gain practical experience in the field of investigation.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [GoogleScholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Santos MR (2024). Conformational Analysis of Arginine Methylated and Di Methylated and Serine Phosphorylated In Silico. J Clin Chem Lab Med. 7:297.
Received: 29-Nov-2024, Manuscript No. JCCLM-24-35796; Editor assigned: 02-Dec-2024, Pre QC No. JCCLM-24-35796 (PQ); Reviewed: 16-Dec-2024, QC No. JCCLM-24-35796; Revised: 23-Jan-2025, Manuscript No. JCCLM-24-35796 (R); Published: 30-Dec-2024 , DOI: 10.35248/2736- 6588.24.7.297
Copyright: © 2024 Santos MR. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.