Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
+44 1478 350008
ISSN: 2161-0398
+44 1478 350008
Research Article - (2014) Volume 4, Issue 1
Natural biopolymers are gaining increasing attention due to their extraordinary affinity towards adsorption of heavy metal ions. In this research, biopolymers such as chitosan and alginate were used to remove contaminants from lean alkanolamine (methyldiethnolamine; MDEA) solvent. The sweetening of natural gas is mostly done by absorption using single or mixed alkanolamine solutions. Alkanolamine solvent quality deteriorates abruptly while absorbing H2S/CO2 from natural gas and causes foaming in natural gas sweetening unit. Different types of organic as well as inorganic species are present as contaminant in lean MDEA solvent. Heat stable salts such as acetate and propionate are present in high amount in lean MDEA solutions and detected using Ion chromatograph and UV-VIS spectrophotometer. Substantial amount of elements (chromium, iron etc.) were detected using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) analysis. Chitosan coated sand (CCS) and calcium alginate (Ca-Alg) beads were prepared and used in batch adsorption studies to monitor the removal of these contaminants in MDEA solvents. FTIR studies and SEM analysis were done to observe the underlying principle of bio-adsorbent preparation. It was observed that both CCS and Ca-Alg removed heavy metal ions like chromium (40% and 61%) and iron (21% and 62%) significantly. Ca-Alg could also remove organic acid anions (36%) appreciably. The maximum uptake capacity for chitosan coated sand was found to be 59.88 μgm/gm for iron and 38.022 μgm/gm for chromium.
<Keywords: Gas sweetening; Contaminants; Heat stable salts; Biopolymer; Adsorption
Natural biopolymers such as chitosan and alginate are gaining momentum due to their extraordinary affinity towards adsorption of heavy metal ions. Particularly in the hydrogel form, the uptake is comparable or even better than that of commercial ion exchange resins. In addition, the materials are abundantly and easily available, biocompatible and environmentally friendly, making them potential bio-sorbents for the removal of pollutants mostly from water and wastewater [1-11]. Of all the biopolymers, chitosan has been studied extensively over the last decade due to its unique properties in adsorbing metal ions from industrial chemical waste. It is one of the naturally occurring biopolymer and derived from N-deacetylation of chitin (the major component of the shells of crustacean organisms). Chitosan is non-toxic, biocompatible, having high chemical reactivity, chirality, chelation and adsorption properties [12-14]. The presence of both amine and hydroxyl groups in chitosan makes it unique among biopolymers and accounts for its affinity towards removal of heavy metal ions such as chromium [1-4], copper [1,5-7], mercury [8,9] and lead [10,11] from aqueous solutions. However, the chitosan beads suffer disadvantages of poor chemical resistance and mechanical strength which significantly reduces the recycle life of the chitosan beads. To improve these properties, chitosan was coated on sand to obtain good mechanical and chemical properties. Alginate (Alg) is a binary heteropolymer obtained from marine algae and contains varying proportions of α-L-guluronic acid (G-block) and β-D-mannuronic acid (M-block) units in a pyranose form, arranged in linear blocks (Figure 1). It is used in diverse fields, such as the pharmaceutical industry [15,16], food industry [17], drug delivery [18], enzyme encapsulation [19] and contrast agent development for diagnostic imaging [20]. Alginates are also proven to be excellent adsorbent for water purification. Researchers have demonstrated that calcium-alginate gel beads can remove heavy metals from industrial wastewater.
Natural gas sweetening using aqueous tertiary alkanolamine such as Methyldiethanolamine (MDEA, approximately 50 weight %) is currently used by Gasco Habshan, Abu Dhabi to remove acid gases like H2S/CO2. In this absorption process formate, acetate, propionate etc. are produced by the reaction between aerial oxygen and H2S and CO2. The reactions between these anionic species (such as formate, thiosulfate, acetate, thiocyanate etc.) and protonated MDEA forms a group of salts known as heat stable salt (HSS), which cannot be removed from the solvent by heating in the regenerator [21,22]. Continuous accumulation of these HSS in the solvent deteriorates its quality making the system less sensitive to the H2S absorption. The increase of HSS accumulation in solution also leads to the corrosion and fouling of the process equipment [23-25]. To maintain a constant concentration of MDEA solution, make-up water and MDEA are added regularly in the process. Due to the continuous running of plants, heavy metal ions as contaminants gets accumulated in the lean MDEA solvent mostly coming from metal corrosion and erosion caused in the process and some from the regularly added make up water. Moreover, the solution foams in the process which causes significant loss of MDEA. Therefore, the regeneration of MDEA is a challenge for the industry. Though there are a few attempts of HSS removal from amine solutions using newer processes like distillation and electro-dialysis [26-28], they suffer from serious drawbacks like cost effectivity, higher energy requirements and continuous supply of huge volumes of water.
Generally, for the removal of total contaminants (HSS as well as metal ions) from industrial lean amine (MDEA) solvent ion exchange resins are used by the industry. MDEA containing both amine and hydroxyl group adhere heavy metal ions strongly. Thus bare ionexchange resins cannot always remove heavy metal ions successfully from lean MDEA solvent overcoming the chelation. Thus, using biopolymers like chitosan and alginate to remove contaminants are first of its kind as no work has been reported previously. The novelty of this research work lies in the preparation of chitosan coated sand (CCS) and calcium alginate (ca-Alg) beads which were used as an adsorbent for the removal of heavy metal ions (mostly chromium and iron) and organic acid anions accumulated in lean MDEA solution in gas sweetening process. FTIR studies and SEM analysis were carried out to study the underlying principle of bio-adsorbent preparation. Finally, the batch adsorption studies for the removal of contaminants were investigated. The standard adsorption isotherm models like, Langmuir and Freundlich were used to verify the mechanism of the adsorption process.
Materials
Lean amine solvents were obtained from the Gasco, Habshan, Abu Dhabi containing 50 weight% methyldiethanolamine (MDEA). Chitosan was purchased from Sigma Chemical Co. (St. Louis, USA). Sodium alginate was obtained from Loba Chemie (Mumbai, India) and used as received. Two ICP standard solutions were obtained from Perkin, Elmer. Sand was collected from the campus area of the Petroleum Institute, Abu Dhabi.
Analysis of organic acid and inorganic anions (HSS)
Organic acid anions were detected in both Dionex IC5000 chromatographic systems and UV-VIS Spectrophotometer (DR5000, Hach Lange). Organic acid anions (e.g. glycolate, formate, acetate, propionate, butyrate, valerate)) were separated and quantified in the lean methyldiethanolamine samples using Ion-Chrmatograph (Dionex-ICS5000). Separate stock standard solutions were prepared for each anion in deionized water. Lean MDEA samples were diluted to calibration range and filtered before injection. The specific conditions for detection of organic acid anions are given in Table 1.
| Parameter | Organic Acid |
|---|---|
| Analytical column | ICE AS1 |
| Eluent | 1 mmol/l Heptafluorobutyric acid |
| Eluent flow rate | 0.8 (ml/min.) |
| Background signal | <115 μS/cm |
| System pressure (psi) | 940 |
| Detection | Suppressed conductivity |
| Suppressor | AMMS ICE300 micro membrane |
Table 1: Specific conditions for detection of Organic acid in Ion-Chromatograph
Fatty acids react with dihydroxy compound in acidic environment forming fatty acid esters. These are reduced by Fe3+ salts to form a red colored complex which was estimated by UV-VIS spectrophotometer (Test kit 365). This was the basis for estimation of total organic acids in lean MDEA solvents.
Analysis of metal ions in inductively coupled plasma optical emission spectrometry
Elemental analysis of metal ions was performed using ICP-OES (Perkin Elmer, Optima 8000). Most of the metal ions in the lean MDEA solutions are accumulated by metal corrosion and erosion in the system and from make-up water.
FTIR and SEM analysis
The FTIR studies of chitosan coated sand (CCS) and calcium alginate (Ca-Alg) beads were carried out using FTIR, Nicolet, iS10, Thermo Scientific instrument using OMNIC software. Surface morphology of the bio-adsorbents was studied using Scanning Electron Microscopy (SEM) with FEI Quanta 200, The Netherland.
Preparation of sand coated with chitosan and calcium alginate beads used as adsorbents
Sand was collected from the campus area of the Petroleum Institute, Abu Dhabi, washed with distilled water and dried. 1.0 gm chitosan powder was added to 100 ml 5% acetic acid solution and left overnight in stirring condition for dissolving it completely. 10 grams of dried sand was added into the chitosan solution and stirred for six hours, decanted and kept at vacuum oven at 60°C for curing. The sand particles was then added drop wise to 100 ml 1 (M) sodium hydroxide solution and stirred overnight for chitosan coating. The chitosan coated sand were dried, washed and used for adsorption studies. After drying, the CCS was sieved and the homogenous particle size of 0.50 mm was used for heavy metal ions adsorption experiments.
Sodium alginate bio-polymeric beads employed as adsorbent were prepared in two steps. In the first step, a known solution of 1 weight % sodium alginate was added drop wise into a 0.1 (M) CaCl2 solution with the help of a syringe and under constant stirring. The beads so produced were allowed to harden by leaving them in CaCl2 solution for 24 hour and thereafter filtered and washed with distilled water. The calcium alginate bio-polymeric beads were stored in distilled water at room temperature and used as such for batch adsorption studies.
Batch ion exchange experiments
The batch adsorption experiments were conducted using mixed batch reactor technique for % removal of metal ions and organic acid anions using chitosan coated sand (CCS) and calcium alginate (Ca- Alg) beads. The experiments were conducted in 100 ml conical flasks containing 10 ml of lean amine solution and known weights of the adsorbent kept under the water bath shaker (Dihan, Korea) at 150 rpm for 24 hours to attain equilibrium. For measuring the residual contaminant concentrations, samples were withdrawn after 24 hours and measured [29]. The amount of contaminants removed using batch equilibrium study was calculated as:
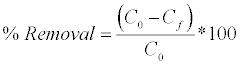 (1)
(1)
Where, C0 and Cf are the respective ion concentrations in solution initially and at equilibrium after 24 hours.
4.7 Adsorption isotherms
Adsorption isotherms are used to study the distribution of adsorbate between solid adsorbent and liquid phase at equilibrium. The adsorbate uptake capacity at equilibrium qe) for various amount of the adsorbent were plotted with equilibrium concentration Ce of the metal ions and fitted to the standard Langmuir and Freundlich, isotherm model.
The Langmuir isotherm [30] assumes uniform monolayer coverage of adsorbate over a homogenous adsorbent surface.
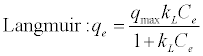 (2)
(2)
Where, qmax is the maximum sorption capacity of adsorbent, Ce is the equilibrium adsorbate concentration and k_L is the corresponding Langmuir isotherm constant.
The Freundlich adsorption isotherm [31] is based on empirical formula and has been interpreted as adsorption to heterogeneous surfaces sites.
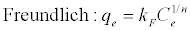 (3)
(3)
In this equation kF and n are the Freundlich constants. This expression is characterized by the heterogeneity factor, n and the value of n determines the adsorption process to be physical or chemical. If the value of n is above unity, the adsorption process is physical adsorption and it shows chemical adsorption for values less than unity [32].
Anionic contaminants
Concentrations of organic acid anions from Ion chromatographic studies are shown in Figure 2. Among all the organic acids present in the lean MDEA solution, acetate (3256 ppm) is present in maximum amount followed by propionate (1727 ppm), butyrate (806.6 ppm), glycolate (303.3 ppm), valerate (254.4 ppm) and formate (227.7 ppm).
The total organic acid content using Test kit 365 was found to be 5737.5 ppm while that obtained from chromatographic separation was found to be 5737.3 ppm. Hence total amount of organic acid content measured using UV-VIS spectrophotometer gave almost same data as measured using Ion-chromatographic analysis. Hence, Test kit 365 was used for all further experiments for estimation of the total organic acid anions [29] present in lean MDEA samples.
Metal ions
The major metal ions present in lean amine (MDEA) samples were chromium (615.7 ppb), iron (1114 ppb), magnesium (75.29 ppb), manganese (111.7 ppb), cadmium (48.32 ppb), strontium (26.81 ppb) and titanium (31.77 ppb) etc. detected using ICP-OES.
FTIR analysis
FTIR studies were carried out to observe the spectrum for chitosan coated sand (CCS) (Figure 3a). The extended spectral band for the chitosan coated sand appears at 3675 cm-1 due to axial OH group and N-H stretch of secondary amine. Peaks are also obtained at 1738.24 cm-1 (C=O stretch of amide), 1417.31 cm-1 (NH angular deformation in CONH plane) and 2360.37 cm-1 (CN asymmetric band stretching). In the spectrum, the plane bending vibration due to the presence of Si-O bond is indicated by the presence of peaks 977 and 1374.46 cm-1 for chitosan coated sand [33]. From FTIR results, it can be observed that chitosan was successfully coated on sand.
For calcium alginate (Figure 3b) the following peaks are observed. The broad peak at 3312.50 cm-1 in the spectrum of alginate is attributed to the hydroxyl groups. While two bands at 1599.53 cm-1 and 1422.99 cm-1 are assigned to the asymmetric and symmetric stretching vibrations of the carboxyl group of alginate molecule respectively [34,35]. Additional sharp peak at 1021.87 cm-1 was due to C-O groups [36]. Thus, stable calcium alginate beads were prepared for adsorption studies.
SEM Analysis
Figure 4a confirmed the presence of many pores and some cracks on the surface while chitosan was coated on sand particles [37]. Similarly, in Figure 4b cracks and small pores are observed as caused by partial collapsing of the polymer network during dehydration. It can be seen that the surface of the gel bead is regular undulating with some scattered wrinkles. This may be explained by ‘egg-box’ structure as guluronate blocks from sodium alginate molecule chains are responsible for this box formation with Ca+2 ions during alginate gelation. Moreover, the biopolymer carrying carboxylic group is capable of forming complexes with metal ions and some metal ions can cooperatively bind between the G-blocks of adjacent alginate chains. Therefore, it can be concluded that the calcium ions preferentially located at the surface of the bead because the surface complex formation involving calcium carboxylate seems to be the mechanism for divalent metal ion cross-linking of the alginate network [38].
Removal of contaminants
Chitosan containing –NH2 groups in the biopolymer backbone can only successfully remove heavy metal ions like chromium and iron from industrial lean MDEA solution and maximum removal of 62% was achieved for metal ions at room temperature (23°C). Whereas, calcium alginate beads can remove both organic acid anions (36% removal was observed) as well as heavy metal ions significantly as it contains both –COOH and –OH groups in the biopolymer backbone (Figure 5).
Equilibrium isotherm studies
The equilibrium MDEA concentration (Ce) for various uptake capacities of heavy metal ions (qe) were fitted to Langmuir and Freundlich isotherm model. The predicted constants from the isotherm for chitosan coated sand are shown in Table 2. The maximum uptake capacity (qmax) at 23°C for iron and chromium ions were found to be 59.88 μg/gm and 38.022 μg/gm, respectively. Thus iron uptake capacity was larger than chromium in CCS. The n values from Freundlich isotherm for both iron and chromium values were more than unity confirmed the physical adsorption process.
| Isotherm | Parameter | Iron | Chromium |
|---|---|---|---|
| Langmuir | qmax(μg/gm) | 59.88 | 38.022 |
| kL(L/gram) | 307.63 | 442.51 | |
| R2 | 0.9945 | 0.9951 | |
| Freundlich | kF | 0.0352 | 0.0264 |
| n | 1.146 | 1.112 | |
| R2 | 0.9944 | 0.9945 |
Table 2: Isotherm model parameters for the adsorption of heavy metal ions on chitosan coated sand.
In this research work, biopolymers like chitosan and alginates were successful used as adsorbent for the removal of metal ions as well as organic acid anions present in industrial lean MDEA samples obtained from Gasco, Habshan (Abu Dhabi). Different types of organic as well as inorganic species are present as contaminant in lean MDEA solvent used in natural gas sweetening process. Heat stable salts such as acetate (3256 ppm) and propionate (1727 ppm) were present in higher amount in lean MDEA solutions as detected using Ion chromatograph. Total organic acid anion of 5737.5 ppm was determined using UV-VIS spectrophotometer. Substantial amount of elements such as chromium (615.7 ppb), iron (1114 ppb) etc. were detected using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) analysis. Chitosan coated sand (CCS) and calcium alginate (Ca-Alg) beads were prepared to check the batch adsorption studies for the removal of the contaminants. It was observed that both CCS and Ca-Alg removed heavy metal ions like chromium (40% and 61%) and iron (21% and 62%) significantly including organic acid anions removal (36%) by Ca-Alg. The equilibrium batch sorption studies for heavy metal ions removal were fitted into Langmuir and Freundlich isotherm models. The maximum uptake capacity for chitosan coated sand was found to be 59.88 μgm/gm for iron and 38.022 μgm/gm for chromium.