Journal of Pollution Effects & Control
Open Access
ISSN: 2375-4397
ISSN: 2375-4397
Research Article - (2021)Volume 9, Issue 4
The contaminants of emerging concern (CECs) have been one of the major concerns in recent. The objective of this study was to find potential CECs using an analytical screening method “comprehensive target analysis with an automated identification and quantification system (CTA-AIQS)” which uses gas and liquid chromatography combined with mass spectrometry (GC-MS and LC-MS). CTA-AIQS was applied to analyze sediment core samples collected from Osaka Bay, Japan, in 2019. Among the 74 chemicals were detected in sediment core samples, six sterols, PPCPs (fexofenadine, diphenhydramine, and clarithromycin), and tris(2-isopropylphenyl)phosphate isomers (TIPPPs) showed increasing profile from bottom to surface layer of the sediment core. Through the screening with chemical properties (persistence, bioaccumulation potential, and toxicity) of those chemicals, TIPPPs were categorized as potential CECs in the marine environment in Japan.
Persistent Organic Pollutants (Pops), Contaminants Of Emerging Concern (Cecs), Automated Identification And Quantification System (Aiqs), Marine Sediment Core; Phosphate Flame Retardants (Pfrs), Tris(2-Isopropylphenyl)Phosphate Isomers (Tippps)
The contaminants of emerging concern (CECs) have been one of the major concerns in recent. CECs have been defined as naturally occurring or anthropogenic chemicals that “have now been discovered or are suspected to be present in various environmental compartments and whose toxicity or persistence is likely to significantly alter the metabolism of a living being”[1]. The screening surveys of potential CECs have been conducted using the model-based technique among more than 100,000 chemicals in commercial use [2-6]. Those potential CECs were classified as various categories as; alkyl phenols; brominated flame retardants (BFRs); perfluorinated compounds; pharmaceuticals and personal care products (PPCPs); current-use pesticides including insecticides, fungicides, and herbicides; single-walled carbon nanotubes; and organophosphate flame retardants (PFRs) [7,8]. Most of these CECs are currently unregulated because their environmental levels, fates, and effects on organisms are poorly understood.
Although numerous numbers of potential CECs have been reported, few compounds have been analyzed as monitoring surveys. Because considerable numbers of chemicals of potential CECs are present in environmental media, and it is not realistic to develop the method of “target analysis” for individual potential CECs. For this reason, a number of research groups have attempted to detect CECs in the environment using the “comprehensive analysis” as screening method with gas and liquid chromatography coupled with high-resolution mass spectrometry (GC-HRMS and LC-HRMS) and the potential CECs have been detected by suspect screening [9-14] and nontarget screening [15-18].
In this study, Comprehensive Target Analysis with an Automated Identification and Quantification System (CTA-AIQS) was used as a tool to detect potential CECs in environment. CTA-AIQS is one of suspect screening technique that can be used to identify and semiquantify approximately 1500 chemicals in environmental media [19]. CTA-AIQS has been applied to assess water and sediment quality in various countries [20-23]. In addition, CTA-AIQS has been used as the first pollution screening scheme on the scene of natural disasters such as earthquakes in Japan [24,25].
In this study, CTA-AIQS was used to analyze sediment core samples for screening the potential CECs in Japanese coastal environment. The vertical profile of chemical concentrations in sediment core samples is expected to be useful for estimating the historical trends of chemical loads in marine environments. The chemical concentrations in sediment core sample showed increasing trend from bottom to surface may indicate the increasing trend of environmental loads of those chemicals. Our research group conducted to find potential CECs by analyzing sediment core samples collected in Beppu Bay, Japan using CTA-AIQS. The results indicated that three polycyclic aromatic hydrocarbons (PAHs; anthracene, chrysene, and fluoranthene) and tris(2-isopropylphenyl)phosphate isomers (TIPPPs) could be categorized as potential CECs in marine environments in Japan [26]. To verify this result, this study planned to analyze sediment core samples collected in Osaka Bay, which receives large volumes of sewage and industrial wastewater flows, because coastal area of Osaka Bay is one of the biggest economic and industrial regions in Japan. Environmental surveys of dioxin-related chemicals and heavy metals using sediment core samples in Osaka Bay have been conducted [27,28]; however, sediment core samples have not previously been analyzed by comprehensive screening to find potential CECs.
The objective of this study was to find chemicals that might be classified as potential CECs by assessing the historical trends of chemical concentrations in sediment core samples collected from Osaka Bay, Japan, by means of CTA-AIQS.
Sample Collection and Sediment Core Dating
Osaka Bay is located on the eastern part of the Seto Inland Sea, central Japan. The sea has a seawater volume of 41 km3, an area of 1,450 km2, an average depth of 28 m, and a maximum depth of 197 m. The Yodo, Yamato, and Muko rivers are the main inflowing rivers, with a total estimated flow of 13.1 km3 yr−1. Osaka Bay is bounded by Osaka and Hyogo prefectures, which contain the largest metropolitan areas in Japan, with populations of 9 million and 5 million people, respectively. Osaka and Kobe are some of the largest international ports in Japan, and a major industrial area is located along the coast. The bay is surrounded by the islands of Honshu and Awaji and is classified as an enclosed coastal sea because it has only two narrow mouths. Water exchange in the bay is therefore limited, and there is potential for accumulation of toxic pollutants associated with human activity. Two sediment cores (OS2-1, core length 84 cm; OS2-2, core length 78 cm) were obtained in Osaka Bay (OS2; 34°35ʹ30.96ʹʹ N, 135°16ʹ7.8ʹʹ2 E; Figure S1) from the research vessel Kaikomaru by an underwater diver using an acrylic pipe (inner diameter = 10 cm) in December 2019. The cores were collected without disturbing the sediment– water interface, held horizontally during transport to a laboratory, and cut into 2-cm layers. The layers were stored at −20 °C until analysis. To obtain samples that were large enough for both comprehensive and target analysis, we combined the sectioned layers to yield eight samples, from depths of 0–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, and 70–78 cm. These samples were dried under flowing nitrogen for several days at room temperature and then stored at −20 °C prior to chemical analysis.
Sediment chronology analysis was performed for the OS2-1 core based on the constant rate of supply (CRS) method of 210Pb dating [29] and verified using the 137Cs peak for the year 1963 [30]. Details of the CRS-based chronological method are provided in the Supplementary Information (Figure S2). Dried samples were sealed in holders for a month to allow 222Rn and its short-lived decay product (214Pb) to equilibrate. The activity of supported 210Pb was estimated by measuring the activity of 214Pb, whereas that of excess 210Pb was determined from the difference between the total and the supported 210Pb (210Pb excess = 210Pb total −214Pb). The 137Cs, 210Pb, and 214Pb activities were determined by gamma counting using a germanium detector (GCW-3523 CANBERRA, Mirion Technologies, Inc., USA) equipped with a multi-channel analyzer at Idea Co. Ltd., Osaka. The age of a given sample mass depth was calculated using the 210Pb excess inventory, which was obtained by numerical integration of the radioactivity of 210Pb excess versus the mass depth profile [30]. The dating of core OS2-2 was estimated indirectly based on stratigraphic correlations of the depth profiles of magnetic susceptibility between cores OS2-1 and OS2-2. For the correlation, the marked peaks in the profiles were used as time-reference horizons. Samples from core OS2-2 were dated by linear interpolation from the depths and ages of two timereference horizons. Magnetic susceptibility was measured using an SM-30 meter (ZH instruments, Brno, Czech Republic).
Sample Preparation for CTA-AIQS
For CTA-AIQS (GC), samples were prepared according to the method previously reported with slight modifications [31,32,24,26]. A mixture of surrogate standard added prior to extraction and mixture of internal standard added before injection were in Table S1. For CTA-AIQS (LC), samples were prepared according to the previously reported method [33-35]. A mixture of surrogate standard added prior to extraction and mixture of internal standard added before injection were in Table S2. The moisture content of sediment samples was calculated by oven-drying an aliquot of the sample at 130 °C overnight.
Instrument for CTA-AIQS
The organic micropollutants in the samples were identified and semiquantified by means of the AIQS scheme using a private database composed of retention times, mass spectra, and calibration curves. The database for AIQS contained 970 chemicals for GC (Table S3) and 501 organic micropollutants for LC-MS (Table S4). The GC-MS conditions (Table S5) have been reported previously [36]. The LC-MS measurement conditions (Table S6) have previously been reported [34,26].
Targeted Analysis of PFRs
Targeted analysis of PFRs was conducted by the method previously reported with slight modifications [37]. Sixteen monomeric PFRs were selected as target compounds (abbreviations are explained in Table S7). Detailed information about the multiple-reactionmonitoring (MRM) transitions is given in Table S7.
Quality Control
For CTA-AIQS, recovery tests for the entire procedure (both GC and LC) have previously been carried out for chemicals with a wide range of structures, functional groups, boiling points, and other physicochemical properties. For CTA-AIQS (GC), model compounds spiked prior to extraction showed recoveries of 69%– 110% in water samples [38,39] and sediment samples [31,32]. When samples were spiked with surrogate standards prior to extraction, recoveries ranged from 69% to 115%, and the relative standard deviation (RSD) was less than 20% [40]. Recovery tests for CTA-AIQS (LC) have also been conducted for water samples and for soil samples [33-35]. When soil samples were spiked with model compounds prior to extraction, recoveries ranged from 76% to 125%. In another study, mean recoveries of surrogate standards in influent and effluent water samples collected from a sewage treatment plant were 77% and 85% (with RSDs < 23%) [34]. Method detection limits (MDLs) for CTA-AIQS (GC and LC) were estimated from instrument detection limits in the AIQS database on the basis of a 2.5-g dry sediment sample and an injection volume of 1 μL (Tables S3 and S4). Because the standard deviations of the procedural blanks for several of the chemicals (e.g., phthalate-related chemicals) were high, these chemicals were not quantified in this study. CTA-AIQS (GC) was validated by analysis of a certified reference material (NIST 1941b), and the values obtained were 50%–150% of the reference values (average 78%, RSD < 20% for each chemical: Table S8).
For target analysis, samples were analyzed in accordance with the established laboratory quality assurance and quality control procedures. Calibration curves obtained from the LC-ESI-MS/ MS data showed good linearity (R> 0.99). Average recoveries of internal standards for all samples ranged from 61% to 75%. The instrumental detection limits of the analytes were calculated from signal-to-noise ratios determined from the LC-ESI-MS/MS chromatographs. The MDLs for the analytes in all the samples are summarized in Table S8. The concentrations of analytes in the procedural blank were below the MDL values.
Statistical Analysis
The concentrations of chemicals in soil samples are expressed in nanograms per gram in dry weight. For calculation of total concentrations, values less than the MDL were considered to be zero. The Wilcoxon rank sum test was applied to evaluate group differences with the JMP software suite (ver. 12, SAS Institute Inc.). In this study, a p value of less than 0.05 was considered to indicate statistical significance.
Chronology in Osaka Bay
The results of 210Pb radioactivity for the chronological OS2-1 core (Figure S3) demonstrated that 210Pb radioactivity in the core declined non-exponentially with depth, implying that sediment accumulation rates are not constant and that the CRS model was the most appropriate for determining sediment age [29]. The CRS-based 210Pb chronologies indicated that the calendar year corresponding to a core depth of 53 cm (layer at 52–54 cm depth) was 1927±17 AD (Figure S4). The calendar age errors in core OS2-1 were estimated to be <2 yr after 1974 and <9 yr after 1944. The slope of the excess 210Pb plot was steeper in the levels below 17 cm than in the higher levels (Figure S5). There was a minor 137Cs pulse at 37–43 cm and large fluctuations in 137Cs between the detection limit and 0.002 Bq g−1 below 47 cm depth (Figure S3). These variations likely resulted from heterogeneous vertical mixing of sediments by macrobenthos. A minor peak of 137Cs was measured at 55 cm (Figure S3), which probably formed as a result of downward transport of sediments with high 137Cs values by macrobenthos. The minor pulse in 137Cs at 37–43 cm is most likely associated with the bomb-derived fall-out maximum in Japan in 1963 [30]. However, the depth corresponding to 1964 based on the CRS age–depth model was 34 cm (Figure S4), 3–9 cm higher than the 137Cs minor pulse. The occurrence of moderate values above the pulse suggests steady-state rapid mixing in the surface layer [41]. Theoretical mixing modeling demonstrated that steady-state rapid mixing in the surface layer redistributes bomb-derived 137Cs both upward and downward, resulting in an alteration of the original vertical profile with a sharp 137Cs pulse toward 3 cm depth and a minor 137Cs pulse together with moderate values in the upper layer [41]. A strong steady-state mixing experiment (surface 6-cm layer) did not yield a sharp 137Cs pulse; instead, it exhibited constant 137Cs values in the upper 7 cm and a rapid decrease in values below that depth. In contrast, in a moderate mixing experiment (surface 3 cm layer) the sharp 137Cs pulse broadened and shifted 2.5 cm downward, and the surface values increased relative to the original vertical profile. Our observed depth profile of 137Cs was consistent with that of a mixing experiment for the surface 3 cm of sediment. Therefore, steady-state mixing of the 3-cm surface layer may produce moderate values of 137Cs in the upper layer together with a 3-cm downward shift of the 137Cs pulse and yield constant excess 210Pb values in the upper 6 or 7 cm of sediment. This finding indicates that the approximately-half thickness of an upper layer with a constant excess 210Pb value corresponds to the thickness of the steady-state mixing and the depth of the downward shift of the 137Cs pulse. Our data show a vertical profile pattern similar to the latter, suggesting moderate surface steady-state mixing at this site. Given the observed constant excess 210Pb values in the upper 12 cm (Figure S5) and the minor 137Cs pulse at 37–43 cm (Figure S3), steady-state mixing probably occurred in the uppermost 6 cm, and the original 137Cs pulse might have been located at 31–37 cm, consistent with the CRS age–depth model (Figure S4).
Profiles of magnetic susceptibilities and pigments (Figure S6) contained four time-reference horizons (Table S9), which were considered to represent contemporary environmental or biological events in the study site of Osaka Bay. We estimated the ages of core OS2-2 based on these four reference layers and the core top (0 cm, 2019.9 AD). The estimated ages for core OS2-2 were 1908.9, 1935.8, 1953.5, and 1993.1 AD at core depths of 63, 47, 37, and 17 cm, respectively.
Comprehensive Analysis with CTA-AIQS
In this study, CTA-AIQS was used for comprehensive screening to detect CECs in samples from a sediment core (OS2-1) collected in 2019 from Osaka Bay, Japan. Among 74 compounds detected,70 compounds were found with CTA-AIQS (GC), and4 compounds were detected by CTA-AIQS (LC) (Figure 1 and Table S10). The median total concentration (range, minimum–maximum) of chemicals detected in sediment core samples was 8100 (1000– 24000) ng g−1 dry weight. The detected chemicals in sediment core samples were classified into four categories (Figure 1 and Table S10): sterols, alkanes, PAHs, and miscellaneous chemicals (including POPs, PPCPs, and industry).
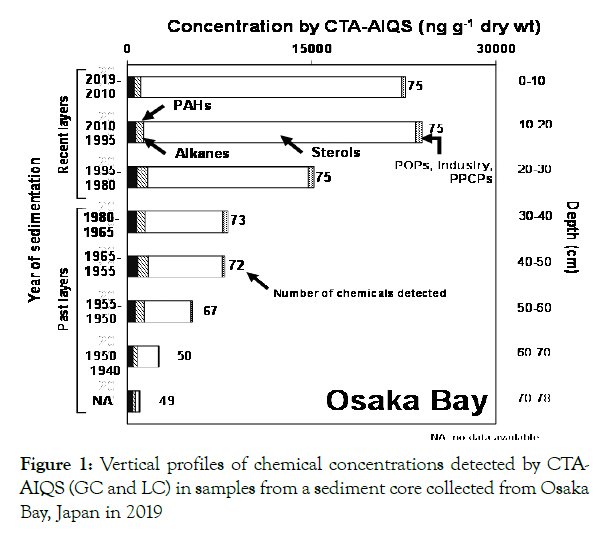
Figure 1. Vertical profiles of chemical concentrations detected by CTAAIQS (GC and LC) in samples from a sediment core collected from Osaka Bay, Japan in 2019
Overview of Vertical Profiles
The total concentration of chemicals detected in sediment samples by CTA-AIQS was lowest in the bottom layers and gradually increased toward the surface layers (Figure 1). In the surface layer, sterols were the predominant chemicals (94%), followed by alkanes (2.5%), PAHs (2.3%), and miscellaneous chemicals (1.4%).
As the first step to discuss the vertical profile of chemical concentrations, POPs must be the important chemical marker to understand appropriateness. The concentrations of POPs, which are known to be CECs, e.g., PCBs, DDTs, and related chemicals, were relatively low compared to those of other types of chemical in this study (Table S10). The vertical profile of POPs in Osaka Bay showed lower concentrations in bottom and surface layers and the highest concentrations in the layers representing the 1930s–1950s (Figure S7). Previous studies of sediment core samples collected from various places in Japan, such as Tokyo Bay, Osaka Bay, Lake Biwa, and Beppu Bay, reported that the highest concentrations of POPs were detected in the layers deposited around the 1970s, because large quantities of these chemicals were used in Japan during the 1960s and 1970s [42-46]. Although concentration profile of POPs in this study seems reasonable, discordance of the sedimentation year on the highest concentration was observed. As discussed above, sediment disturbance by biological and anthropogenic activity might be the reason for this discordance in this study.
Sterols were the chemical category with the highest concentration in this study (Figure 1). Six sterols (cholesterol, cholestanol, campesterol, stigmasterol, beta-sitosterol, and stigmastanol) were detected in all layers (Table S10). Almost the same sterols were detected in marine surface sediments collected from Dokai Bay, Tokyo Bay, and Beppu Bay, Japan [31,47]. The sterol concentrations in this study are comparable to those in Dokai Bay and Tokyo Bay, but 10 times higher than those in Beppu Bay. Sterols and related chemicals in marine and lake sediments can be derived from sewage effluent, but also from terrestrial plants, phytoplankton, and animal feces [48-50]. The higher concentrations of sterols in Osaka Bay than in Beppu Bay are consistent with the large amount of sewage effluent loading from urban areas. The vertical profile of sterol concentrations in this study showed gradually increasing from bottom to surface (Figure S8). A similar increasing trend of sterol concentrations was reported in sediment core samples collected in Beppu Bay. These results suggest a historical increase in sewage effluent loading from urban areas surrounding Osaka Bay. Additionally, degradation of sterols in the deeper layers due to their short half-lives (e.g., cholesterol < 40 days in sediment) might be another reason for the decline in sterol concentrations with depth [51].
Alkanes exhibited the second highest concentration in the sediment core samples in this study (Figure 1). The 25 alkanes (C9–C33) were detected in all layers (Figure S9). The essentially uniform alkane concentrations in all layers yielded a consistent vertical profile. Because alkanes can be derived from various sources, several molecular markers are often used to estimate their origins. The carbon preference index (CPI), which is the ratio of odd-to-even carbon number n-alkanes, was applied to differentiate petrogenic and biogenic alkanes. A CPI value of less than 2 indicates a petrogenic (oil) source; a value greater than 2 indicates a biogenic source [52-55]. The CPI range in Osaka Bay was 1.9–2.5, with a value of less than 2 for most levels (Figure S10). This result suggests that the alkanes detected in Osaka Bay could have been derived from petrogenic sources, which, because Osaka Bay is surrounded by urban and industrialized areas, seems reasonable. The uniform vertical profiles in Osaka Bay indicate continuous input of alkanes from petrogenic sources.
PAHs were detected in all layers of the sediment core samples, and 22 species were identified (16 native PAHs and 6 methylated PAHs; Table S10). The vertical profiles of PAHs concentrations were relatively uniform in all layers (Figure S11). For estimation of the major sources of PAHs, the ratio of the concentration of methylated phenanthrenes to those of phenanthrene (ΣMPhen/ Phen) was applied. A ΣMPhen/Phen value of more than 1 indicates a petrogenic (oil) origin; a value less than 1 suggests a pyrogenic (combustion) source [56]. The average ΣMPhen/Phen ratios in the sediment core sample in this study were approximately 1, with a value of more than 1 for several layers (Figure S12). This result suggests that the PAHs in Osaka Bay are derived mainly from pyrogenic sources but partly from petrogenic sources. Additionally, shorter HL of methylated PAHs than the native PAHs [57] might be another reason for lower value of this ratio in deeper layers. This conclusion is plausible because Osaka Bay is a major industrial and urbanized area in Japan, and the result for PAHs is consistent with that for alkanes.
The concentrations of Miscellaneous (PPCP, and industry) were relatively low compared to those of other types of chemicals in Osaka Bay (Figure 1). Concentrations of PPCPs (including clarithromycin, fexofenadine, and diphenhydramine) and industrial chemicals (including TIPPPs and 4-methyl-2,6-di-tbutylphenol) were gradually increased from the bottom to the surface of the sediment profile (Figures S13 and S14). Although no research has been conducted on these chemicals in sediment core samples, this result might suggest a recent increase in the input these substances into the marine environment from sewage effluent. TIPPPs were also reported to exhibit an increasing trend in sediment core samples collected from Beppu Bay [26]. Detailed profiles of individual chemicals are discussed in the next section.
Screening of Potential CECs
In this study, 74 compounds were identified and semiquantified in sediment core samples using CTA-AIQS (GC and LC). Among these chemicals, screening was conducted to select potential CECs following a three-step protocol.
In step 1, sediment core layers were divided into two groups, “past layers” and “recent layers”, to compare the chemical concentrations of the two groups. The deeper five layers (<1920–1970) were defined as “past layers”, which included the high consumption of POPs around the 1970s in Japan. The shallower three layers (1970–2019) were defined as “recent layers”, which included the generation of post-POPs (Figure 1). In step 2, chemicals exhibiting significantly higher concentrations in recent layers were statistically selected as candidates (p< 0.05, Wilcoxon’s rank sum test). In step 3, among the candidate chemicals, the properties of Persistence, Bioaccumulation, and Toxicity (PBT) criteria were used to select potential CESs. The PBT criteria has been applied to identify chemicals with a high impact on the environment, with high persistence, bioaccumulation, and toxicity: half-life in soil/ sediment > 180 days, bioconcentration factor (BCF) > 2000, and toxicity (NOEC) < 0.01 mg L−1 [58], respectively.
Through the steps 1 and 2, 11 compounds were selected (Table 1): 2,6-dimethylnapthalene, six sterols (cholesterols, beta-sitosterol, stigmasterol, campesterol, cholestanol, and stigmastanol), TIPPPs, and three PPCPs (fexofenadine, diphenhydramine, and clarithromycin). The vertical profiles of one representative chemical for each category are shown in Figure 2. For the selected chemicals, screening by using the PBT criteria (step 3) was conducted (Table 1). For sterols, no reliable data on PBT criteria were found, although their half-lives and toxicity for laboratory animals were shorter and lower than the criteria [59]. Assessment surveys of dimethylnapthalene reported that its halflife in sediment/water, NOEC, and BCF on aquatic ecosystems were <14 days, 0.19 mg L−1, and 700–1200, respectively [60,61]. According to our assessment, cholesterols and PAHs did not meet the PBT criteria (Table 1). Assessment surveys of clarithromycin reported that the BCF calculated by the EPA/BCFWIN system and the NOEC for Daphnia magna were 56 [62] and > 2100 μg L−1 [63], respectively, but no report was found for environmental halflife. For fexofenadine, a BCF of 43.9 for Daphnia pulex[64] and an NOEC on aquatic ecosystems of >330–570 mg L−1 [65] have been reported, but we could find no information on the environmental half-life. A BCF of 4.2–53.3 for fathead minnows [66] and a 10- day NOEC for Daphnia magna of 0.00294 mg L−1 [67] have been measured for diphenhydramine, but there is no information on its environmental half-life. According to these assessments, these PPCPs do not satisfy the PBT criteria (Table 1); however, because information for these chemicals is limited, further investigations of their environmental behavior and PBT properties are required. The remaining compound in Table 1 was TIPPPs. The reported half-life in sediment, BCF, and chronic NOEC for Daphnia magna of TIPPPs are >180 days[68], >2000 [69], and <0.01 mg L−1 [70], respectively. These results indicate that TIPPPs meet the PBT criteria [68]; thus, on the basis of our three-step screening protocol, TIPPPs can be identified as potential CECs (Table 1).
| Compounds | Increasing trend | *Persistence | Bioaccumulative | Toxicity | Potencial of CECsReference |
|---|---|---|---|---|---|
| PAHs | |||||
| 2,6-Dimethylnapthalene | ✓ | x | x | x | a), b) |
| Sterol | ✓ | ||||
| Cholesterols |
✓ | x | x | x | j), k) |
| Beta-sitosterol | ✓ | na | na | na | |
| Stigmasterol | ✓ | na | na | na | |
| Campesterol | ✓ | na | na | na | |
| Cholestanol | ✓ | na | na | na | |
| Stigmastanol | ✓ | na | na | na | |
| Industry | |||||
| Tris(2-isopropylphenyl)phosphate (TIPPPs) | ✓ | ✓ | ✓ | ✓ | c), l), m) |
| PPCPs | |||||
| Fexofenadine | ✓ | na | na | na | d), e) |
| Diphenhydramine | ✓ | na | na | ✓ | f), g) |
| Clarithromycin | ✓ | na | na | na | h), i) |
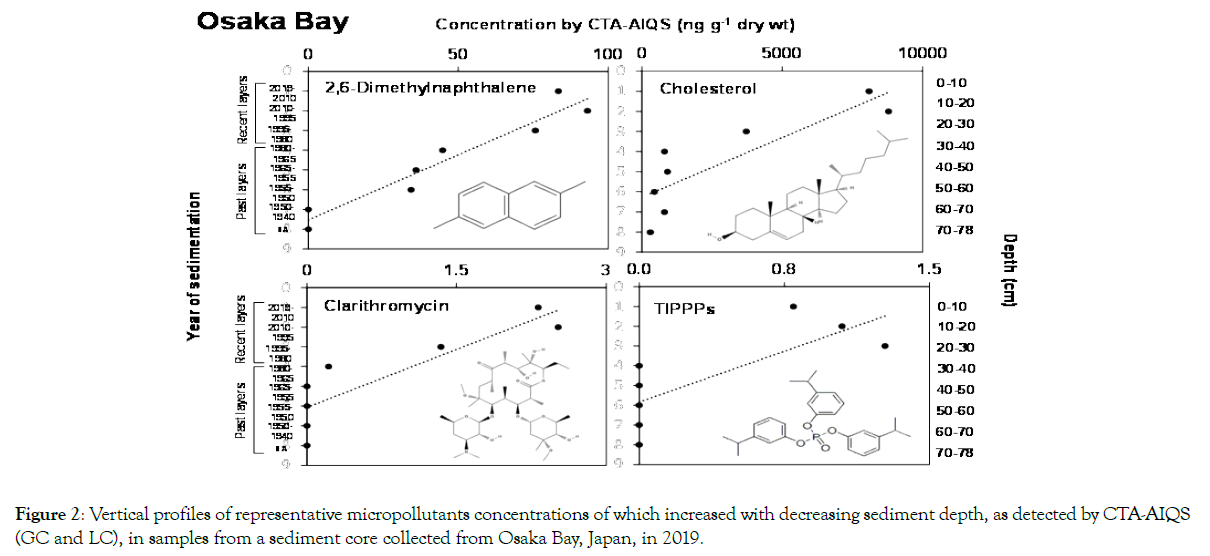
Figure 2. Vertical profiles of representative micropollutants concentrations of which increased with decreasing sediment depth, as detected by CTA-AIQS (GC and LC), in samples from a sediment core collected from Osaka Bay, Japan, in 2019.
TIPPPs as Potential CECs
By applying the protocol described above, TIPPPs can be categorized as potential CECs; however, there are limitations to the sensitivity and accuracy of CTA-AIQS as an analytical method. To confirm the existence of TIPPPs with higher sensitivity and accuracy, target analysis for PFRs by using LC-MS/MS on the same sediment core samples in this study. From this analysis, several PFRs (EHDPP, TDMPP, TIPPPs, and TEHP) were detected. The concentration of TIPPPs ranged from <MDL to 2.2 ng g−1 dry (Table S11). Although the range of TIPPPs concentrations in Osaka Bay was lower than those in Beppu Bay [26], the vertical profile of TIPPPs in Osaka Bay exhibited increasing trend from bottom to surface which was similar to Beppu Bay (Table S11). These results indicate a ubiquitous distribution and increasing load of TIPPPs in coastal environments of Japan. However, halogenated PFRs (e.g., TCEP and TDCPP) were not detected in sediment core samples from Osaka Bay in this study and also from Beppu Bay by. Halogenated PFRs can become concentrated as CECs in samples of water and organisms, and they were one of the most abundant CECs analyzed in previous studies [71,72]. Because halogenated PFRs have relatively higher water solubility than other PFRs, these chemicals do not accumulate in sediment.
In general, increasing trend of PFR consumption has been reported since the use of BFRs (tetra to heptabromodiphenyl ether formulations in 2009, hexabromocyclododecane in 2013, and decabromodiphenyl ether in 2017) was restricted by the Stockholm Convention [73-75]. Since penta-BDE formulations were phased out, TIPPPs contained in Firemaster 550 and ITP organophosphate aryl ester technical mixtures have increasingly been used to treat polyurethane foam in residential upholstered furniture [76]. The environmental monitoring data for TIPPPs are limited. However, TIPPPs were detected in air [77], house dust [78,79], sediment, and bivalves.
In addition, the POP-like characteristics of TIPPPs (overall persistence and [80, 81] long-range transport potential) were evaluated by using the OECD Pov and LRTP Screening Tool [82,83]. TIPPPs showed the values near or above the two lines for the POP criteria (Figure S15; White and gray keys denote partition coefficient data from EPI-Suite ver. 4.11 and UFZ-LSER Database, respectively. Vertical and horizontal lines denote POP criteria for Pov and CTD or TE, respectively. (Pov (days) is a measure of the time scale of degradation of the chemical in the whole environment. CTD (km) and TE (%) represents a transport-oriented metric and a target-oriented indicator, respectively. Details of these parameters are described in an earlier paper. The tool requires the three degradation half-lives in air, water, and soil, and also two partition coefficients such as 1-octanol/water partition coefficient (Kow) and air/water partition coefficient (Kaw) for the chemical of interest. The half-lives were estimated using EPI-Suite ver. 4.11 (https:// www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-programinterface). The log Kow and log Kaw values were estimated using EPIsuite ver. 4.11 and UFZ-LSER Database [84]. Since TIPPPs might have POPs potential (persistence and long-range transport), those
Comprehensive analytical method of CTA-AIQS (which combines GC and LC with MS) was applied to detect CECs from a sediment core collected in Osaka Bay, Japan, in 2019. Among the chemicals detected, TIPPPs concentrations showed increasing trend from bottom to top layer among the core samples. Through the screening processes, TIPPPs were categorized as potential CECs in marine environments in Japan.
This study was supported by the Environment Research and Technology Development Fund (SII-3-1 and -2: JPMEERF18S20310 and 18S20320) from the Environmental Restoration and Conservation Agency of Japan (ERCA). The study was also partially supported by Grants-in-Aid for Scientific Research (17K00528 and 20H00646) and Joint Usage/Research Center–Leading Academia in Marine and Environment Pollution Research (LaMer) from the Japan Society for the Promotion of Science (JSPS). We thank Dr. Isao Watanabe; Ms. Kana Kadota; the staff and students of CATE and CMES (Ehime University); and Ms. Mino Hasegawa and Mr. Humiaki Kato from the National Institute for Environmental Studies, Japan, for valuable support with sampling and analysis of the samples.
Funding
This study was supported by the Environment Research and Technology Development Fund (SII-3-1 and -2: JPMEERF18S20310 and 18S20320) from the Environmental Restoration and Conservation Agency of Japan (ERCA). The study was also partially supported by Grants-in-Aid for Scientific Research (17K00528and 20H00646) and Joint Usage/Research Center–Leading Academia in Marine and Environment Pollution Research (LaMer) from the Japan Society for the Promotion of Science (JSPS), by the fund from the Takahashi Industrial and Economic Research Foundation (10-003-386), and by the fund from the Agriculture & Livestock Industries Corporation, Japan (1714).
Conflicts of Interest/Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Citation: Nishimuta K, Ueno D, Takahashi S, Kuwae M, Tsugeki NK, Kadokami K, et al. (2021) Contaminants of Emerging Concern Detected By Comprehensive Target Analysis in a Sediment Core Collected From Osaka Bay, Japan. J Pollut Eff Cont 9:283.
Received: 09-Mar-2021 Accepted: 20-Mar-2021 Published: 27-Mar-2021 , DOI: 10.35248/2375-4397.21.9.283
Copyright: �©2021 Nishimuta K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was supported by the Environment Research and Technology Development Fund (SII-3-1 and -2: JPMEERF18S20310 and 18S20320) from the Environmental Restoration and Conservation Agency of Japan (ERCA). The study was also partially supported by Grants-in-Aid for Scientific Research (17K00528and20H00646) and Joint Usage/Research Center�¢??Leading Academia in Marine and Environment Pollution Research (LaMer) from the Japan Society for the Promotion of Science (JSPS), by the fund from the Takahashi Industrial and Economic Research Foundation (10-003-386), and by the fund from the Agriculture & Livestock Industries Corporation, Japan (1714).