Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2024)Volume 12, Issue 12
This article evaluates the impact of D-dimer on Venous Thromboembolism (VTE) in cancer patients through a systematic review of the literature. The study searched the PubMed, Embase, and Cochrane databases from 2009 to 2023 and conducted a meta-analysis following PRISMA guidelines. The results indicated that D-dimer significantly increased the risk of VTE (HR: 1.29; 95% Confidence Interval (CI): 1.11-1.47, I² = 34.8%). A total of 23,658 patients were included in the analysis of 32 eligible studies, demonstrating moderate heterogeneity among the studies (I²=34.8%). This review not only elucidates the relationship between d-dimer and VTE risk but also underscores the potential clinical utility of this molecular marker. Despite certain methodological limitations, the findings provide a substantial body of scientific evidence to inform future medical decisions and prevention strategies.
Aim: A systematic review of the literature assessed the influence of d-dimer levels on the development of venous thrombosis (VTE) in patients with cancer.
Methods and results: Between 2009 and 2023, the articles were searched for in PubMed, Embase, and the Cochrane Library, including all relevant studies, and the search strategy has been implemented. This meta-analysis was conducted in accordance with PRISMA, Preferred Reporting Items for Systematic Literature Review and Meta-Analysis. The Odds Ratio (OR), Hazard Ratio (HR), and 95% CI were then calculated and pooled by means of a random effect model. The statistical diversity was evaluated by using I². A comprehensive review was carried out of 32 eligible studies for the pooling of data in our trial. The total number of patients was 23658 and the characteristics. D-dimer significantly increased the risk of VTE [HR: 1.29; 95% CI, 1.11-1.47, I²=34.8%]. Between studies, there was a moderate degree of heterogeneity (I²=34.8%).
Conclusion: D-dimer is an efficient predictor of VTE risk, which should be considered as a classification and promising tool to assess the risk of thromboembolic events in patients with cancer.
D-dimer, Biomarker, VTE, Cancer, Venous thromboembolism
The main risk factor for VTE is cancer [1]. Elevated D-dimer is associated with increased risk for VTE associated with cancer [2]. Patients with tumors have a 4-7 times higher risk of developing venous thrombotic events [3,4]. Therefore, the use of biomarkers should be used to predict and guide the management of cancer-related VTE. VTE is the second most frequently preventable cause of death in cancer patients, after infection [5]. VTE, including DVT and pulmonary embolism [6]. The incidence of cancer is steadily rising in the EU, and improvements in cancer survival and a changing age structure mean that more people now suffer from this co-existing condition. Furthermore, cancer treatments like surgery and chemotherapy also increase the risk of VTE [7].
D-dimer levels increase due to fibrosis and fibrinolysis. D-dimer is a marker of the hypercoagulable phase and is a product of fibrin degradation [8]. Plasma D-dimer measurement has been widely used for screening VTE [9]. In order to comprehensively examine the existing evidence, we carefully evaluated various published studies that have examined the relationship between D-dimer levels and the risk of VTE. This included both qualitative analyses and quantitative assessments of the associations between D-dimer level and VTE events. Our aim was to discern if there were consistent patterns or significant outliers in the data that could lend credence to either a causal relationship or a limited impact from D-dimer on VTE risk prediction in cancer populations. Additionally, we sought to determine if the findings could be generalized across different types of cancer patients, such as those with different stages of disease, surgical procedures, or chemotherapy treatments.
Through our extensive study, we anticipate to identify any gaps in the literature that might have been overlooked, thereby ensuring that our analysis provided a comprehensive picture of the current state of knowledge regarding the role of D-dimer in predicting VTE risks among cancer individuals. By synthesizing the vast array of evidence, we aimed not only to refine our understanding but also to contribute to the advancement of clinical decision making in this critical domain.
Study identification
Between 2009 and 2023, the articles were searched for in PubMed, Embase, and the Cochrane Library, including all related studies, and the search strategy was implemented in Table 1.
| PubMed | |
| #1 | ("Neoplasms"[MeSH Terms] OR ("Tumor"[Title/Abstract] OR "Neoplasm"[Title/Abstract] OR "Tumors"[Title/Abstract] OR "Neoplasia"[Title/Abstract] OR "Neoplasias"[Title/Abstract] OR "Cancer"[Title/Abstract] OR "Cancers"[Title/Abstract] OR "malignant neoplasm"[Title/Abstract] OR "Malignancy"[Title/Abstract] OR "Malignancies"[Title/Abstract] OR "malignant neoplasms"[Title/Abstract] OR "neoplasm malignant"[Title/Abstract] OR "neoplasms malignant"[Title/Abstract] OR "benign neoplasms"[Title/Abstract] OR "benign neoplasm"[Title/Abstract] OR "neoplasms benign"[Title/Abstract] OR "neoplasm benign"[Title/Abstract])) ("Neoplasms"[MeSH Terms] OR ("Tumor"[Title/Abstract] OR "Neoplasm"[Title/Abstract] OR "Tumors"[Title/Abstract] OR "Neoplasia"[Title/Abstract] OR "Neoplasias"[Title/Abstract] OR "Cancer"[Title/Abstract] OR "Cancers"[Title/Abstract] OR "malignant neoplasm"[Title/Abstract] OR "Malignancy"[Title/Abstract] OR "Malignancies"[Title/Abstract] OR "malignant neoplasms"[Title/Abstract] OR "neoplasm malignant"[Title/Abstract] OR "neoplasms malignant"[Title/Abstract] OR "benign neoplasms"[Title/Abstract] OR "benign neoplasm"[Title/Abstract] OR "neoplasms benign"[Title/Abstract] OR "neoplasm benign"[Title/Abstract])) |
| #2 | ("d dimer fibrin"[Title/Abstract] OR "d dimer fragments"[Title/Abstract] OR "fibrin fragment d1 dimer"[Title/Abstract] OR "fibrin fragment dd"[Title/Abstract] OR "D-dimer"[Title/Abstract] OR "fibrin fragment d dimer"[Title/Abstract]) |
| #3 | ("Thromboses"[Title/Abstract] OR "Thrombus"[Title/Abstract] OR "blood clot"[Title/Abstract] OR "blood clots"[Title/Abstract] OR "Atherothrombosis"[Title/Abstract] OR "cancer related thrombosis"[Title/Abstract] OR "CAT"[Title/Abstract] OR "deep vein thrombosis"[Title/Abstract] OR "DVT"[Title/Abstract] OR "pulmonary embolism"[Title/Abstract] OR "pulmonary embolisms"[Title/Abstract] OR "PE"[Title/Abstract] OR "venous thromboembolism"[Title/Abstract] OR "VTE"[Title/Abstract]) |
| #4 | #1 AND #2 AND #3 |
| EMBASE | |
| #1 | cancer:ab,ti |
| #2 | 'malignant neoplasm'/exp |
| #3 | 'tumor' OR 'neoplasm' OR 'tumors' OR 'neoplasia' OR 'neoplasias' OR 'cancer' OR 'cancers' OR 'malignant neoplasm' OR 'malignancy' OR 'malignancies' OR 'malignant neoplasms' OR 'neoplasm, malignant' OR 'neoplasms, malignant' OR 'benign neoplasms' OR 'benign neoplasm' OR 'neoplasms, benign' OR 'neoplasm, benign' |
| #4 | 'd-dimer fibrin' OR 'd-dimer fragments' OR 'fibrin fragment d1 dimer' OR 'fibrin fragment dd' OR 'd-dimer'/exp OR 'd-dimer' OR 'fibrin fragment d-dimer' |
| #5 | 'thromboses' OR 'thrombus'/exp OR 'thrombus' OR 'blood clot'/exp OR 'blood clot' OR 'blood clots' OR 'atherothrombosis'/exp OR 'atherothrombosis' OR 'cancer-related thrombosis' OR 'cat'/exp OR 'cat' OR 'deep vein thrombosis'/exp OR 'deep vein thrombosis' OR 'dvt' OR 'pulmonary embolism'/exp OR 'pulmonary embolism' OR 'pulmonary embolisms' OR 'pe'/exp OR 'pe' OR 'venous thromboembolism'/exp OR 'venous thromboembolism' OR 'vte' |
| #6 | #3 AND #4 AND #5 |
| Cochrane library | |
| #1 | MeSH descriptor: [Thrombosis] explode all trees |
| #2 | (Thromboses OR Thrombus OR Blood Clot OR Blood Clots OR Atherothrombosis OR Cancer-related thrombosis OR CAT OR Deep vein thrombosis OR DVT OR Pulmonary embolism OR pulmonary embolisms OR PE OR Venous thromboembolism OR VTE):ti,ab,kw (Word variations have been searched) |
| #3 | #1 or #2 |
| #4 | (D-dimer fibrin OR D-dimer fragments OR fibrin fragment D1 dimer OR fibrin fragment DD OR D-dimer OR fibrin fragment D-dimer):ti,ab,kw (Word variations have been searched) |
| #5 | #3 and #4 |
| #6 | MeSH descriptor: [Neoplasms] explode all trees |
| #7 | (Tumor or Neoplasm or Tumors or Neoplasia or Neoplasias or Cancer or Cancers or Malignant Neoplasm or Malignancy or Malignancies or Malignant Neoplasms or Neoplasm, Malignant or Neoplasms, Malignant or Benign Neoplasms or Benign Neoplasm or Neoplasms, Benign or Neoplasm, Benign):ti,ab,kw (Word variations have been searched) |
| #8 | #6 or #7 |
| #9 | #5 and #8 |
Table 1: Search strategy.
Inclusion criteria: 1) patients aged 18 or over with cancer; 2) VTE risk was predicted by D-dimer; 3) Prospective Studies; 4) Complete Studies (No Comments, Case Reports, Narrative Reviews); 5) capable of deriving HR, OR, RR, with 95% CI [9].
Exclusion criteria: 1) in animals or in vitro; 2) less than 20 patients (to reduce the error rate); 3) cancer patients <18 years of age; 4) pregnant women.
Search policy is made without any language restrictions. For possible other eligible studies, all references included in this retrospective study were screened (Table 1).
Study selection
Two authors (Jiayuan Li, Lei Zhang) have independently reviewed all selected titles and abstracts. They have been excluded if the title or abstract is inappropriate for the purpose of our examination. Subsequently, the complete texts of eligible studies were received where the significance of the article could not be ruled out with certainty. The issue was settled by consensus and the third reviewer (Jianjun Sun), where appropriate, the selected studies were eligible if they fulfilled the following criteria: Prospective and retrospective studies were included. Reviews, case reports, and non-human studies were excluded.
Data extraction and quality assessment
While the use of qualitative or qualitative criteria in observational studies is controversial, the quality of the study was evaluated in the cohort study according to the following criteria: type of study (prospective or retrospective); selection of patients (follow-up patients with no possible bias in selection); and control group (age and gender). Give one point for each item that matches. The ascorbate system has been modified in such a way that three quality categories have been identified as follows: a score of 3 is considered a high-quality study, a score of 2 or less is considered a low-quality study, and the total number of people lost to follow-up (<5%, >20%, or 5%-20%) was identified as an additional quality. The following endpoints evaluated the quality of the study for case–control studies: the choice of the patient (a prospective patient with no possible bias); the control group (subsequent inclusion or gender matching). Two points defined high-quality research; one or less defined alpha quality study. The total number of cases was also identified as an additional quality item. VTE was the primary outcome of our analysis. In the majority of the inclusion studies, D-dimer levels were measured once at entry into the study. The choice of the Prisma Flow chart for study was described in Figure 1.
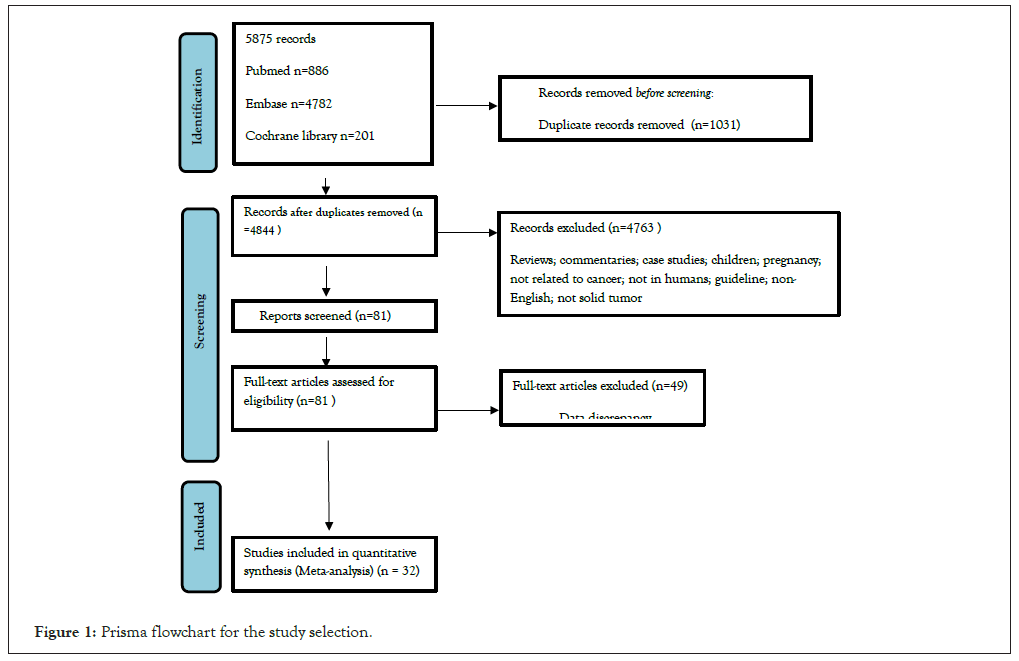
Figure 1: Prisma flowchart for the study selection.
The relevant data extracted from the proposed table, such as the name of the originator, country of origin, publication year, cancer type, sample size, age, gender, the method or time of detection of the D-dimer, the type of anticoagulant, the HR (SHR, OR, or RR) 95% CI, the duration of follow-up, and the D-dimer cut-off.
Statistical analysis
The OR, HR, and 95% CI were calculated. The HR or OR analysis and the 95% confidence intervals were based on the for VTE random effect model of the Mantel- Haenszel. Using I², meta-regression and Cochran Q-test, the statistical heterogeneity was evaluated. Statistical diversity has been evaluated by I², which measures the suitability of combining the results of the trials [10]. I² gives an estimation of the number of variances across the study because of heterogeneity, not coincidence. I², 30% indicates mild heterogeneity, 30%-50% moderate, and 50% severe heterogeneity [11]. In the process of further research, the source of heterogeneity is revealed by subgroup analysis [12]. Presence of publication bias was explored using-based plots of effect size against standard error [13].Study identification and selection (Table 2).
| Study | Country | Sample size |
Recent anti-tumor therapy | Age | Male (%) | D-dimer detection method | Follow-up duration | Risk estimates (95% CI) | Time and method |
D-dimer Cutoff |
|---|---|---|---|---|---|---|---|---|---|---|
| Arpaia et al. [14] | Italy | 124 | Chemotherapy | 63.5 ± 9 | 63 (50.8) | Venous blood samples | 6 months | HR=4.05 (1.22-13.44) |
Time-dependent auto-analyzer |
>650 ng/ml |
| Ay et al. [15] | Austria. | 821 | Chemotherapy Surgery Radiotherapy |
62 (53.0-68.0) |
451 (55.0) | Venous blood samples | 501 days (255-731) | HR=1.80 (1.00-3.24) |
enrollment auto-analyzer |
- |
| Cui et al. [16] | China | 234 | Surgery | 50.0 ± 9.3 | - | Venous blood samples | 12 months | HR= 2.904 (1.046–8.060) | before surgery, one month, three months, six months, and twelve months | 0.55 mg/l |
| Faille et al. [17] | France | 140 | Chemotherapy Surgery Radiotherapy |
58.87 | 84 (60) | Venous blood samples | 6 months | HR=4.9 (1.0–23.1) |
enrollment | ≥ 2.16 µg/ml |
| Ferroni et al. [18] | Italy | 45 | Chemotherapy | 60±9 (36–78) | 28 (62) | Venous blood samples | 12 months | HR=0.4 (0.08–2.70) |
enrollment | - |
| Hiraide et al. [19] | Japan | 682 | Surgery | 67.0 (29–87) | 449 (65.8) | Venous blood samples | 217 (1–1040) days | OR=4.84 (2.62–8.91) |
- | 1.95 mg/ml |
| Jin et al. [20] | China | 160 | Surgery Chemotherapy Radiotherapy Immunotherapy Targeted therapy |
65.1l±l10.4 | 121-75.6 | Venous blood samples | HR=8.449 (4.323-18.536) | - | >5 mg/l | |
| Junjun et al. [21] | China | 106 | Surgery Chemotherapy Targeted therapy Radiation |
77.3 ± 10.9) | 57-53.8 | Venous blood samples | 23.7 months (12.0–62.0 months) | OR=2.73 (1.25–5.96) |
- | ≥ 600ug/l |
| Kawaguchi et al. [22] | Japan | 395 | surgery chemotherapy radiation therapy corticosteroid usage |
18–89 (53) | 212-53.7 | Venous blood samples | - | OR=5.99 (2.46–14.57) |
- | >1.0 mg/dl |
| Kodama et al. [23] | Japan | 267 | Surgery Heparin warfarin |
54 (16–85) | 0 | Venous blood samples | - | OR=1.19 (1.04–1.37) |
before the operation and on certain postoperative days | 5 ug/ml |
| Li et al. [24] | China | 84 | Chemotherapy | - | 38l(45.2) | Venous blood samples | - | OR=8.373 (1.810-38.747) |
enrollment | >0.5 ug/ml |
| Li et al. [25] | China | 198 | Surgery | 47.8l ± l8.1 | 0 | Venous blood samples | 12 months | HR=9.707 (2.611-36.080) |
before surgery 1, 3,6,12month After surgery |
0.94 ug/ml |
| Li et al. [26] | China | 124 | - | 81(65.3) | Venous blood samples | - | OR=1.620, (1.220, 2.152) | - | 1.14 mg/l | |
| Libourel et al. [27] | Netherlands | 126 | - | 69 (65-74) | 63(50) | Venous blood samples | 478 days (range, 0-109 months) | HR=7.66 (1.07-54.80) | - | >4.0 mg/l |
| Ma et al. [28] | ||||||||||
| China | 90 | - | - | 60l(66.7) | Venous blood samples | - | OR=3.1 (1.79–4.32) |
- | - | |
| Mauracher e al. [29] | Austria | 946 | - | 62(52–69) | 503(53.2) | Venous blood samples | 2 years | HR=1.31 (1.00-1.73) (per 10ug/ml) |
enrollment | - |
| Nakamura et al. [30] | Japan | 46 | chemotherapy | 71 (49– 86) | 30 (65.2) | - | 18.2 months. | HR=8.33 (2.57– 27.1) | - | ≥ 15.6 μg/ml |
| Obermeier et al. [31] | Austria | 795 | - | 62 (53-68) | 431(54.2) | - | Median 730 days |
HR=1.02 (0.85-1.21) |
- | - |
| Ohsumi et al. [32] | Japan | 993 | Surgery Chemotherapy Hormone therapy Radiation therapy |
58.4 ± 13.2 | 3(0.3) | Venous blood samples | 1 year | HR=2.61 (0.29–23.43) |
Enrollment | >1.2μg/ml |
| Okusaka et al. [33] | Japan | 1006 | - | 67.6 (69.0) ± 9.8 | 565 (56.2) | Venous blood samples | 1 year | HR=2.03 (0.96–4.31) |
- | >1.2μg/ml |
| Reitter et al. [34] | Japan | 112 | - | 62.4 21.3–80.3 | 64 (57.1) | venous blood samples | 250 days | HR=1.76 (1.32-2.35) |
Enrollment | - |
| Park et al. [35] | South Korea | 241 | Chemotherapy | - | 169 (70.1) | - Auto-analyzer |
11 months (10-12) |
HR=1.32 (1.00-1.75) | enrollment | - |
| Posch et al. [36] | Austria | 167 | - | 62.7 (53.3-68.8) | 94 (56.0) | Venous blood samples | Maximum 250 days |
HR=2.78 (1.69-4.58) (per doubling) |
Time-dependent auto-analyzer |
Continuous |
| Shim et al. [37] | Korea | 3147 | Surgery | 52.5 ± 12.90 | - | Venous blood samples | Mean 5.3 months |
OR=1.037 (0.989–1.087) | - | - |
| Shoji et al. [38] | Japan | 9965 | - | 68 (57–75) | 5,317 (53.4) | Venous blood samples | - | OR=8.165 (3.950-16.87) |
- | ≥ 1.47 µg/ml |
| Stender et al. [39] | Denmark | 176 | Surgery | 68.0 (33.0-94.0) | 114 (65.0) | Plasma Auto-analyzer |
Maximum One year |
HR=6.53 (1.58-27.0) | enrollment Auto-analyzer |
0.30 mg/l |
| Thaler et al. [40] | Austria | 141 | Surgery Chemotherapy Radiotherapy |
54 (43.0-65.0) | 94 (66.7) | plasma | Maximum 2 years | HR=1.33 (1.05-1.66) | enrollment auto-analyzer |
- |
| Tian et al. [41] | China | 552 | Surgery | 53 (46, 61) | - | Venous blood samples | - | OR=3.26 (1.49-7.10) |
- | - |
| Wang et al. [42] | China | 208 | - | 52.6 (23-83) | 0 | Venous blood samples | 746 (2-1680) | HR=1.144 (1.020-1.283) | - | |
| Zhang et al. [43] | China | 369 | Antitumor therapy | 60.05 ( ± 9.24) | 181-49.1 | Venous blood samples | - | OR=4.083, (2.238-7.447) | - | - |
| Zhao et al. [44] | China | 328 | Surgery | 52.9 ± 12.57 | 0 | Venous blood samples | - | OR=15.092; (8.281‑31.353) | - | - |
| Zhou et al. [45] | China | 870 | - | 61.32 ± 10.76 | 368-42.3 | Venous blood samples | - | OR= 1.028 (0.990–1.068) |
- | 2.56ug/L |
Note: HR: Hazard Ratio; OR: Odd’s Ratio; CI: Confidence Interval.
Table 2: Characteristics of included studies.
We identified 5869 potentially relevant studies from the EMBASE database, 4782 from PubMed, and 201 from the Cochrane Library. Sift through references and find 6 relevant articles as additional sources. We ruled out 1,031 duplicate trials. We excluded 4763 studies after title and abstract screening with pre-defined inclusion and exclusion criteria. The other 81 studies were obtained incomplete for a thorough assessment. Of the 81 studies collected, 49 were excluded due to data inconsistencies. Thus, 32 studies were included in this systematic review. The identification of the study and the selection process are presented in Figure 1.
Study characteristics
Table 3, lists the baseline characteristics of enrolled patients. The studies ranged from 45 to 9,636 patients, with a total of 23,658 enrolled patients. In most articles, the association of D-dimer with thrombotic risk has been assessed by applying cutoff values expressed as increased risk above a limit.
Our findings, including 19 HR and 13 OR studies, indicated that elevated serum levels of D-dimer predict increased risk of VTE (HR, 1.29; 95% CI, 1.11-1.47) (Table 3).
| Study ID | Study type | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|---|
| Arpaia et al. [14] | Cohort study | 4 | 1 | 2 | 7 |
| Ay et al. [15] | Cohort study | 4 | 1 | 3 | 8 |
| Cui et al. [16] | Cohort study | 4 | 1 | 3 | 8 |
| Faille et al. [17] | Cohort study | 3 | 0 | 2 | 5 |
| Ferroni et al. [18] | Case control study | 3 | 2 | 2 | 7 |
| Hiraide et al. [19] | Case control study | 4 | 2 | 2 | 8 |
| Jin et al. [20] | Case control study | 4 | 2 | 1 | 7 |
| Junjun et al. [21] | Cohort study | 4 | 2 | 3 | 9 |
| Kawaguchi et al. [22] | Cohort study | 4 | 2 | 3 | 9 |
| Kodama et al. [23] | Case control study | 4 | 2 | 3 | 9 |
| Li et al. [24] | Case control study | 4 | 1 | 3 | 8 |
| Li et al. [25] | Cohort study | 4 | 0 | 3 | 7 |
| Li et al. [26] | Case control study | 4 | 2 | 3 | 9 |
| Libourel et al. [27] | Cohort study | 4 | 1 | 2 | 7 |
| Ma et al. [28] | Case control study | 4 | 2 | 3 | 9 |
| Mauracher e al. [29] | Cohort study | 4 | 1 | 3 | 8 |
| Nakamura et al. [30] | Case control study | 4 | 2 | 3 | 9 |
| Obermeier et al. [31] | Cohort study | 4 | 1 | 2 | 7 |
| Ohsumi et al. [32] | Cohort study | 4 | 1 | 2 | 7 |
| Okusaka et al. [33] | Cohort study | 4 | 1 | 2 | 7 |
| Reitter et al. [34] | Cohort study | 4 | 1 | 1 | 6 |
| Park et al. [35] | Cohort study | 4 | 2 | 3 | 9 |
| Posch et al. [36] | Cohort study | 4 | 2 | 1 | 7 |
| Shim et al. [37] | Case control study | 4 | 0 | 3 | 7 |
| Shoji et al. [38] | Case control study | 4 | 1 | 2 | 7 |
| Stender et al. [39] | Cohort study | 4 | 1 | 3 | 8 |
| Thaler et al. [40] | Cohort study | 4 | 1 | 2 | 7 |
| Tian et al. [41] | Case control study | 4 | 1 | 3 | 8 |
| Wang et al. [42] | Case control study | 4 | 0 | 3 | 7 |
| Zhang et al. [43] | Case control study | 4 | 1 | 3 | 8 |
| Zhao et al. [44] | Case control study | 4 | 2 | 3 | 9 |
| Zhou et al. [45] | Cohort study | 4 | 1 | 2 | 7 |
Table 3: Assessment of bias with Newcastle-Ottawa scale.
Study quality
A summary of the quality evaluation is given in Table 3. I²=34.8%; HR: 1.29, CI: 1.11-1.47), although there was a moderate degree of heterogeneity in the pooled trials (Figures 2 and 3). Funnel scenarios indicated a publication bias for overall VTE outcomes (Egger p<0.001 and Begg (p=0.183) (Figures 4 and 5). Thus, we used a trim-fill approach to remove publication bias, and the HR revealed that the seven additional studies be included and pooled: HR 1.53; 95% CI, 1.72-2.00, which suggests the robustness of the study (Figure 6).
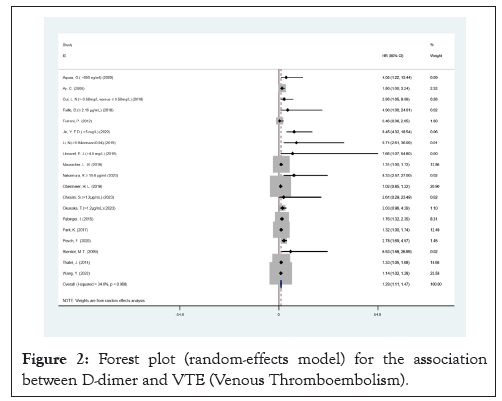
Figure 2: Forest plot (random-effects model) for the association between D-dimer and VTE (Venous Thromboembolism).
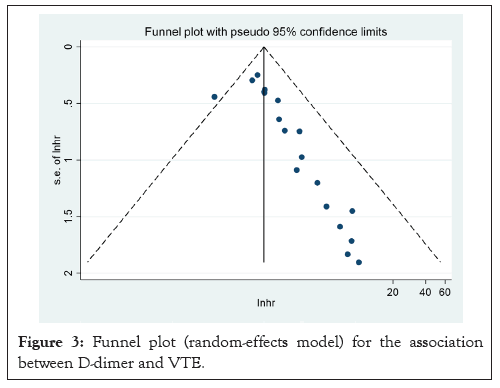
Figure 3: Funnel plot (random-effects model) for the association between D-dimer and VTE.
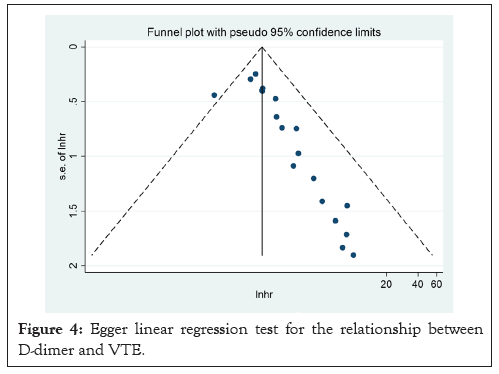
Figure 4: Egger linear regression test for the relationship between D-dimer and VTE.
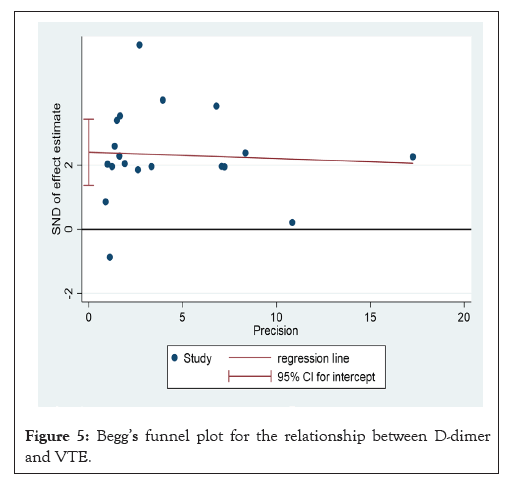
Figure 5: Begg’s funnel plot for the relationship between D-dimer and VTE.
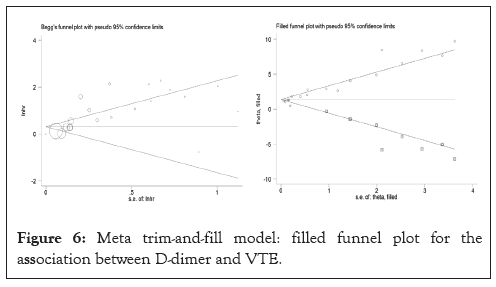
Figure 6: Meta trim-and-fill model: filled funnel plot for the association between D-dimer and VTE.
Test of heterogeneity
In this study, a moderate degree of heterogeneity was observed. We conducted subgroup analysis and meta-regression to identify the sources of heterogeneity as thoroughly as possible. The results indicated that treatment (p=0.913), patient age (p=0.031), region (p=0.565), publication year (p=0.487), maximum follow-up (p=0.249) were relevant factors. Notably, patient age emerged as the primary source of heterogeneity.
Bias of publication
In this research, the visualization funnel plot displayed significant asymmetry, suggesting a potential publication bias (Figure 3).
D-dimer
In a randomized model analysis, it was discovered that elevated D-dimer level significantly increased the risk of VTE (HR, 1.29 95% CI, 1.11-1.47 Figure 2). There was medium heterogeneity between the studies (I²=34.8%, p<0.05), which was due to the case-control cohort studies. No significant change in overall heterogeneity was observed from one study to the next. Looking at the results and the heterogeneity by study design, there were no significant differences between cohort and case-control studies in OR and in I² (Figure 2). Funnel plot is shown in Figure 3, which indicate the publication of bias (Figures 7-9).
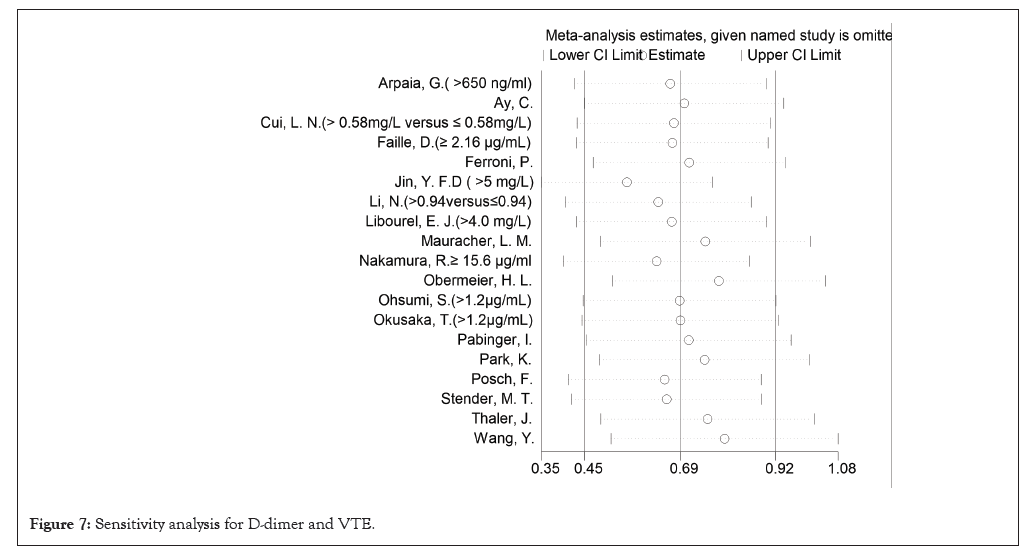
Figure 7: Sensitivity analysis for D-dimer and VTE.
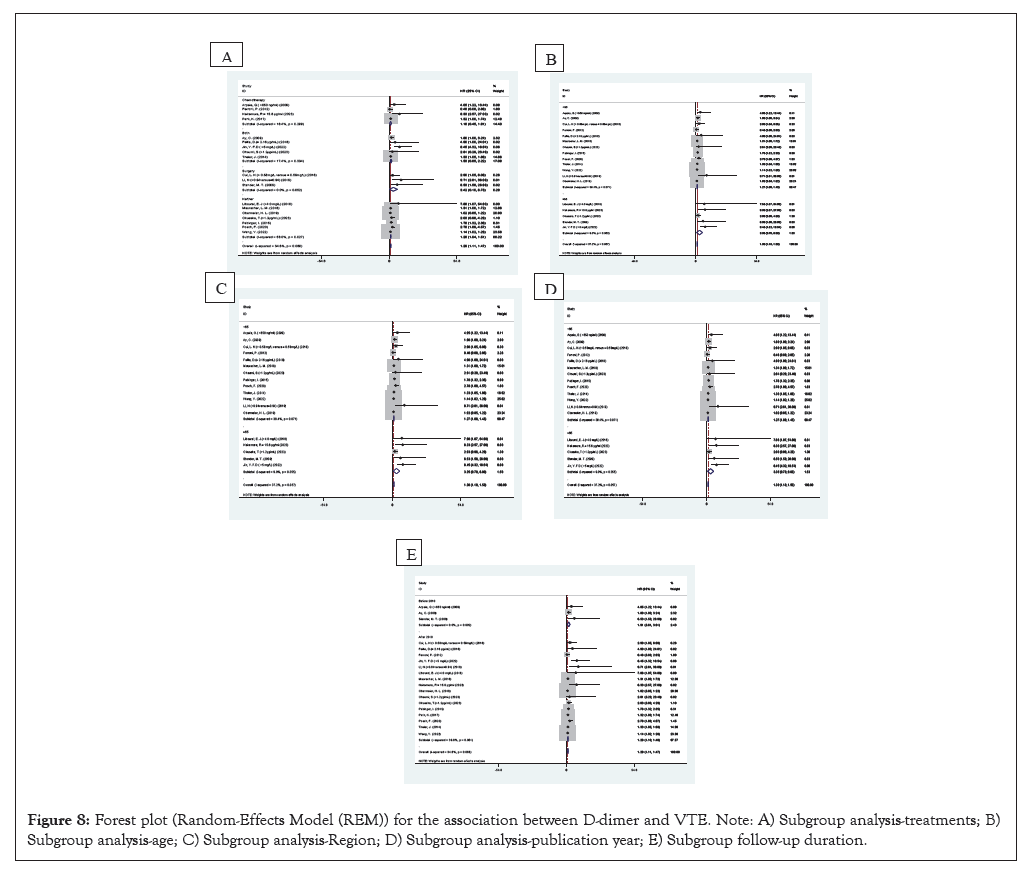
Figure 8: Forest plot (Random-Effects Model (REM)) for the association between D-dimer and VTE. Note: A) Subgroup analysis-treatments; B) Subgroup analysis-age; C) Subgroup analysis-Region; D) Subgroup analysis-publication year; E) Subgroup follow-up duration.
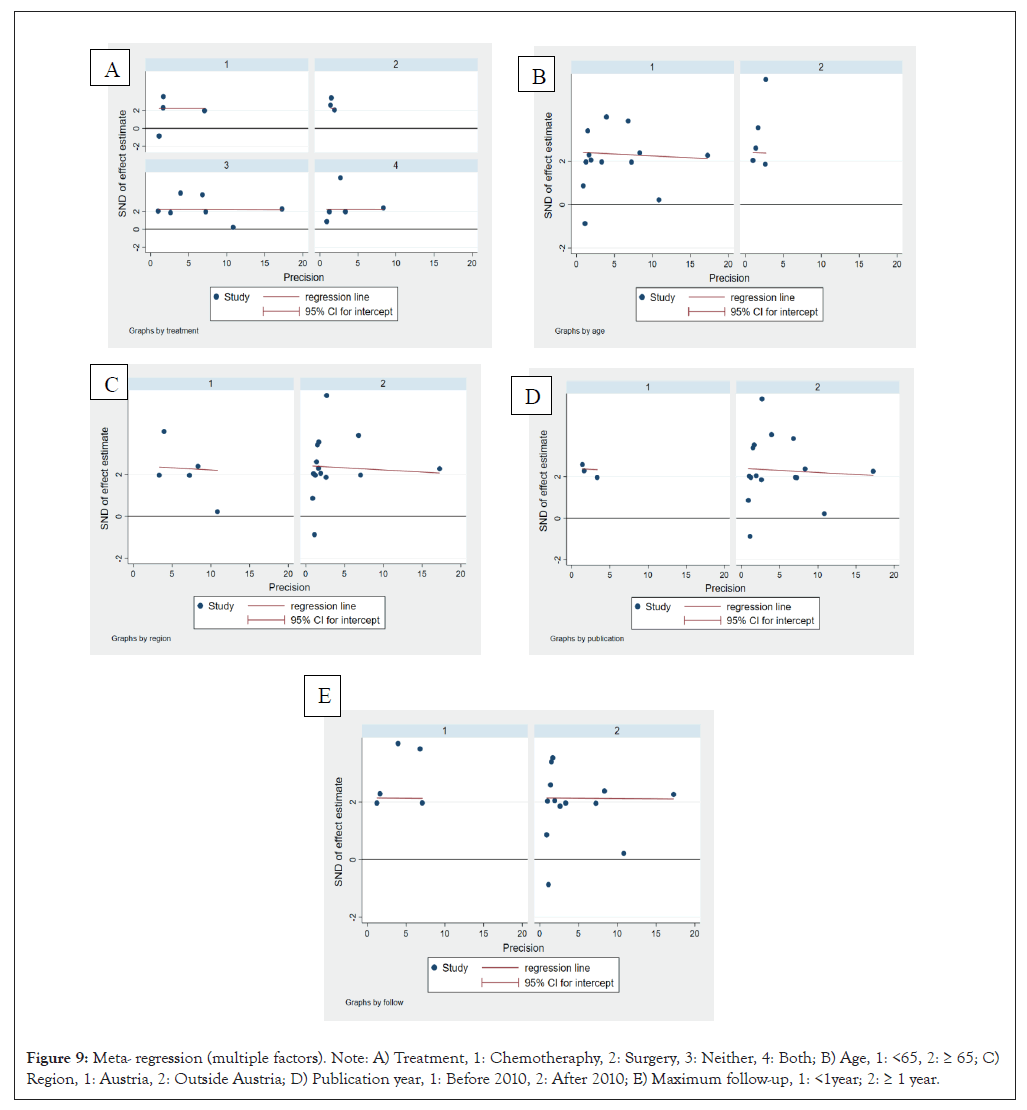
Figure 9: Meta- regression (multiple factors). Note: A) Treatment, 1: Chemotheraphy, 2: Surgery, 3: Neither, 4: Both; B) Age, 1: <65, 2: ≥ 65; C) Region, 1: Austria, 2: Outside Austria; D) Publication year, 1: Before 2010, 2: After 2010; E) Maximum follow-up, 1: <1year; 2: ≥ 1 year.
Bannow’s research revealed the association of D-dimer with VTE in hematological malignancies, and D-dimer levels are only predictive if they are very high or very low (Figures 10 and 11) [46].
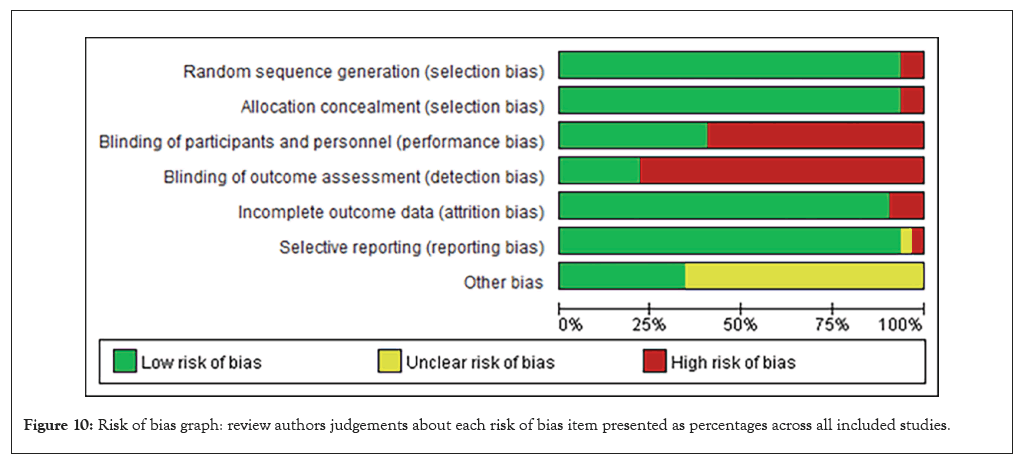
Figure 10: Risk of bias graph: review authors judgements about each risk of bias item presented as percentages across all included studies.
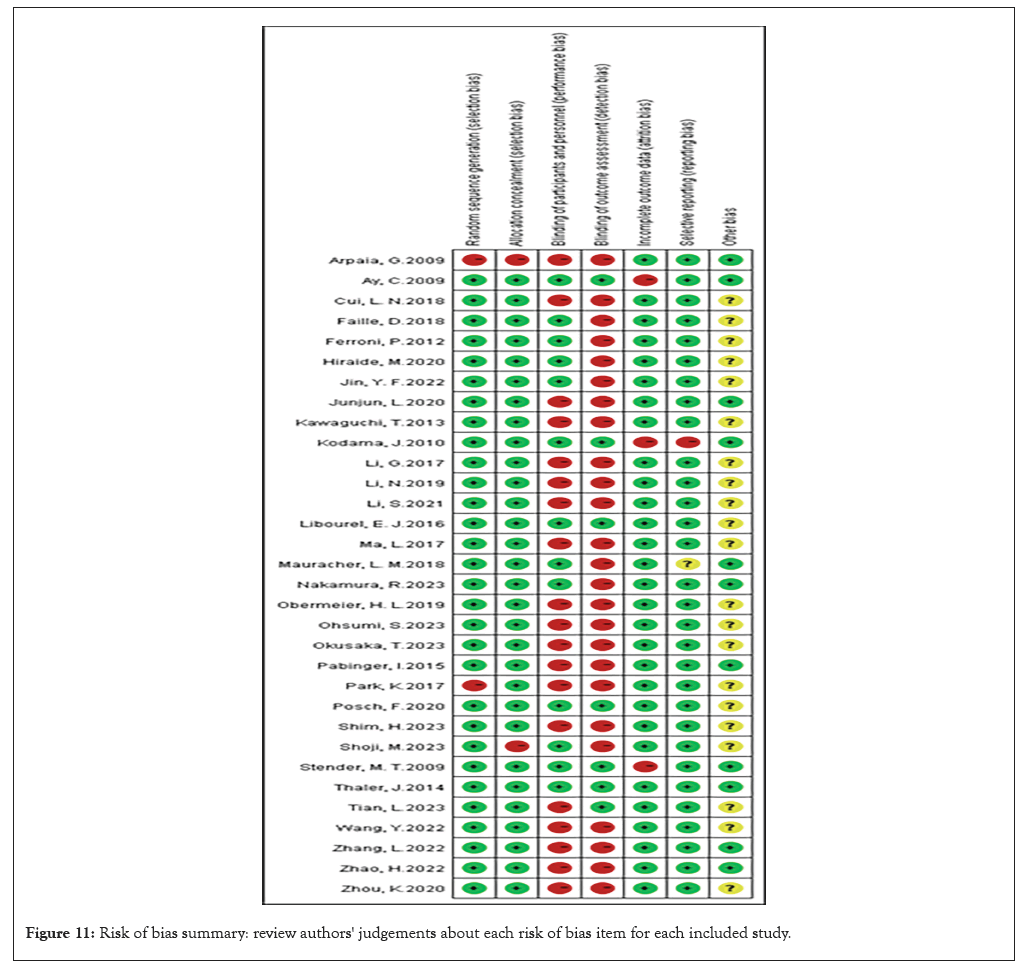
Figure 11: Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
This comprehensive meta-analysis revealed that D-dimer could predict an increased risk for VTE. Furthermore, Meta-HR confirmed that D-dimers may be predictive of an increased risk of VTE even with chemotherapy. These findings reinforced the conclusion that D-dimer might be a potential marker for thromboprophylaxis in the course of chemotherapy. In the surgical cancer patients, the Meta-HR analysis provided preliminary positive results, which were still doubtful, since only five studies were included. In the context of VTE trials, we have noted that some studies have exclusively reported a relationship between a single tumor type and D-dimer levels. Conversely, other studies have differentiated among various tumors and tumor sites, highlighting the specificity inherent in tumor biology. Importantly, different cancer sites may contribute to heterogeneity; thus, future research should investigate whether D-dimer can serve as a predictive marker for VTE across various cancers and consider its integration into international diagnostic scoring systems (Table 4). The small-sample study effect was assessed using Egger’s test, with the results presented in Table 5.
| Study | Stage (%) | Metastases (%) |
|---|---|---|
| Arpaia et al. [14] | - | Total 46% |
| Ay et al. [15] | - | Total 39.9% VTE 40.3% |
| Cui et al. [16] | DVT I/II 87.7% III/IV 12.3% | - |
| Faille et al. [17] | - | Total 59.5% VTE 100% |
| Ferroni et al. [18] | - | - |
| Hiraide et al. [19] | Total: I/II 2.9% III/IV 97.1% VTE I/II 4.2% III/IV 95.8% | Total 70% VTE 63.4% |
| Jin et al. [20] | Total I/II 18.5% III/IV 81.5% DVT I/II 3.8% III/IV 96.2% | - |
| Junjun et al. [21] | PE I/II 30.2% III 43.4% IV 26.4% | - |
| Kawaguchi et al. [22] | Total I 5.8% II 17.2% III 24.6% IV 50.6% VTE III/IV 88.4% | - |
| Kodama et al. [23] | - | - |
| Li et al. [24] | PE I/II 7.1% III/IV 92.9% | - |
| Li et al. [25] | DVT I 29.4% II 70.6% | - |
| Li et al. [26] | - | Total 70.2% VTE 66.7% |
| Libourel et al. [27] | - | - |
| Ma et al. [28] | PE I/II 10% III/IV 90% | - |
| Mauracher e al. [29] | Total I 10.8% II 21.8% III 22.3%IV 45.2% | - |
| Nakamura et al. [30] | Total III/IV 89.1% PE III/IV 80% | PE 90% |
| Obermeier et al. [31] | - | Total 39.1% VTE 41.1% |
| Ohsumi et al. [32] | Total II 73.4% III 16.6%IV 10% VTE II 60% III20% IV20% | - |
| Okusaka et al. [33] | Total II 38.2% III 20.4% IV 41.5% VTE II 19.8% III 10.5% IV 69.8% | Total 39.9% VTE 70.9% |
| Reitter et al. [34] | - | Total 41.1% |
| Park et al. [35] | - | - |
| Posch et al. [36] | - | Total 44% VTE 50% |
| Shim et al. [37] | - | - |
| Shoji et al. [38] | Total 0 to II 45.8% III to IV 27.9% VTE 0 to II 29.6% III to IV51.5% | - |
| Stender et al. [39] | Total I/II 77% III/IV 23% DVT I/II 78%III/IV 22% | - |
| Thaler et al. [40] | - | - |
| Tian et al. [41] | - | - |
| Wang et al. [42] | Total I 20.7% II 8.2%III53.8% IV 17.3% | - |
| VTE I 22.6%II 3.2%III 48.4% IV 25.8% | ||
| Zhang et al. [43] | - | VTE 55.3% |
| Zhao et al. [44] | VTE I +II 50% III+IV 50% | VTE 20% |
| Zhou et al. [45] | - | - |
| Note: DVT: Deep Vein Thrombosis; VTE: Venous Thromboembolism | ||
Table 4: Cancer stage or percentage of patients with metastases in the included studies.
| Stratified analyses | Subgroup analysis | Number of studies | HR | 95% CI | I² (%) | p value (heterogeneity) | Meta-regression (multiple factors) | Egger’s test P value |
|---|---|---|---|---|---|---|---|---|
| 3 | ||||||||
| Time to recent anti-tumor treatment | Chemotherapy | 4 | 1.18 | [0.45-1.91] | 18.40 | 0.299 | 0.503 | |
| Surgery | 3 | 3.42 | [0.10-6.73] | 0.00 | 0.652 | 0.315 | ||
| Neither | 7 | 1.28 | [1.04-1.51] | 58.00 | 0.027 | 0.028 | ||
| Both | 5 | 1.53 | [0.85-2.22] | 17.40 | 0.304 | 0.218 | ||
| Age | 1 | 0.031 | ||||||
| <65 | 13 | 1.27 | [1.08-1.45] | 39.40 | 0.071 | 0.038 | ||
| ≥ 65 | 5 | 3.35 | [0.70-6.00] | 9.00 | 0.355 | 0.602 | ||
| Region | 5 | 0.565 | ||||||
| Austria | 5 | 1.3 | [1.00-1.60] | 60.40 | 0.039 | 0.044 | ||
| Outside Austria | 15 | 1.38 | [1.05-1.66] | 25.20 | 0.182 | 0.001 | ||
| Publication year | 7 | 0.487 | ||||||
| Before 2010 | 3 | 1.91 | [0.81-3.01] | 0.00 | 0.602 | 0.057 | ||
| After 2010 | 16 | 1.28 | [1.10-1.45] | 39.80 | 0.051 | 0.001 | ||
| Maximum Follow-up | 9 | 0.249 | ||||||
| <1year | 5 | 1.66 | [1.18-2.14] | 32 | 0.208 | 0.101 | ||
| ≥ 1 year | 13 | 1.27 | [1.11-1.43] | 28 | 0.13 | 0.002 |
Table 5: Stratified analyses of pooled HR of D-dimer and venous thromboembolism.
After conducting an in-depth analysis of the relationship between D-dimer and VTE, we found that although there are some challenges, such as potential publication bias as revealed by funnel plots, and the data as pointed out by Egger and Begg tests Uncertainty in interpretation. However, by employing trim-and-fill model and careful sensitivity analysis, we were able to confirm the robustness of the overall findings. This robustness suggests that this study is robust in presenting its conclusions despite possible methodological flaws in earlier studies or the lack of published, negative result to provide a more solid evidence base.
This robustness is due to researcher’s in-depth understanding of statistical principles and careful attitude towards data interpretation. Even so, any potential bias should be taken seriously and corrected in future studies. We should remain alert to errors caused by small samples or imperfect study designs and explore the possibility of improving methodologies. At the same time, there is also a need to ensure that all researchers follow uniform methodological standards to avoid duplication of errors and improve research quality. In addition, more researchers are encouraged to share their findings so that other researchers can conduct independent verification based on this knowledge, further reducing the risk of publication bias.
Tumors produce various coagulation factors that promote the formation of thrombin, leading to a hypercoagulable state in cancer patients. Consequently, these biomarkers may be present in clinical settings in future studies.
In this meta-analysis, we completed a comprehensive assessment of D-dimer's predictive increased risk of VTE. This comprehensive study not only provides an insight into the relationship between D-dimer and VTE risk, but also reveals the potential value of this molecular marker in clinical practice. The results of this study provide important scientific evidence for future medical decisions and prevention strategies. Because of a number of methodological limitations, this conclusion needs to be carefully considered, and further, specially designed RCTs should be considered. This systematic review and meta-analysis evaluate the relationship between D-dimer levels and the risk of VTE. Our findings indicate that elevated D-dimer levels are associated with an increased risk of VTE.
We cannot estimate the optimal critical value of D dimer that would effectively predict cancer-related VTE with standard diagnostic accuracy. Due to the unavailability of data, we could not obtain substantial quantities of both true and false positive results, as well as negative ratings. The selection of D-Dimer cutoffs was infrequently based on a standardized method for estimating diagnostic value. Consequently, our design revealed varying cutoffs identified for assessing the role of D-Dimer. In order to determine the D-Dimer cut-off values and improve the accuracy of diagnosis, it is essential to analyze the data collected from a large group of patients and to establish the criteria for inclusion. Moving forward, we will continue to investigate the impact of D-Dimer levels on thrombosis across different cancer types.
Every member of this research team invested equal effort and attention in the overall design concept of the project and in-depth analysis and interpretation of the data. Together they participated in the process from initial conception to final documentation, ensuring that the quality and reliability of the research results were safeguarded. On this basis, each author also personally reviewed and approved the manuscript of the paper to ensure the accuracy and completeness of the research content. Through this collaborative model, we ensure that the research results fully reflect the contributions and expertise of each author. The contributions of each author are outlined as follows: Conceptualization: Jiayuan Li; Data Curation: Jiayuan Li and Lei Zhang; Formal Analysis: Jiayuan Li and Yuan Wang; Methodology: Jiayuan Li and Ying Dong; Project Administration: Jiayuan Li; Resources: Jianjun Sun; Software: Zhanli Guo and Xiaoting Hu; Supervision: Jiayuan Li and Ruoning Li; Visualization: Jiayuan Li and Zhanli Guo; Writing-Original Draft: Jiayuan Li; Writing-Review and Editing: Jiayuan Li and Jianjun Sun.
Authors declare there is no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Li JY, Zhang L, Wang Y, Guo ZL, Hu XT, Li RN, et al. (2024). Correlation between D-dimer and Cancer-associated Thrombosis: A Systematic Review and Meta-analysis. Angiol Open Access. 12:530.
Received: 22-Nov-2024, Manuscript No. AOA-24-35339; Editor assigned: 25-Nov-2024, Pre QC No. AOA-24-35339 (PQ); Reviewed: 09-Dec-2024, QC No. AOA-24-35339; Revised: 16-Dec-2024, Manuscript No. AOA-24-35339 (R); Published: 23-Dec-2024 , DOI: 10.35841/2329-9495.24.12.530
Copyright: © 2024 Li J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.