Advancements in Genetic Engineering
Open Access
ISSN: 2169-0111
ISSN: 2169-0111
Research Article - (2023)Volume 12, Issue 2
The Corona Virus Disease 2019 (COVID-19) pandemic began in 2019; however, its repercussions continue to plague humans in 2023 with its short-term and long-term complications and new variants. One of the complications of the disease is its effect on human reproductive tissues, the treatment of which is crucial for the reproduction of future generations. This review explored the relationship between male and female reproductive tissue dysfunction and Severe Acute Respiratory Syndrome Coronavirus 2(SARS-CoV-2) using genetic data analysis and sequence alignment of COVID-19 patients. The alignment of a specific sequence in the virus variants and five human genes directly involved in gametogenesis, oogenesis, and fertility is interesting. The coronavirus can directly or indirectly interfere with the function of the identified genes.
Dysfunction; Tissue; Genes; Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Viral strains typically evolve in response to temporal and environmental factors. The quantity and quality of the virus or its products may increase or decrease due to these genetic and product modifications. The SARS-CoV-2 virus has undergone many genetic and functional changes since the beginning of its global outbreak, leading to variations in disease severity, disease spread rate, complications, and mortality. According to the World Health Organization (WHO) statistics, the number of confirmed cases was 118964, and the number of deaths was 700 worldwide as of January 23rd, 2023 (https://COVID19.who.int/). The decreased mortality can be attributed to the widespread use of various preventative measures, such as quarantine, infection control, vaccines, and medications. However, hidden morbidity and mortality resulting from COVID-19 complications persist. Male and female reproductive organs such as the testes, ovaries, and placenta are vulnerable to SARS-CoV-2 infection, as they contain Angiotensin-Converting Enzyme 2 (ACE2) receptor and express ACE2 receptor activity [1]. This virus utilizes this enzyme to enter human cells, as the testis is rich in ACE2 [2]. Moreover, some studies have demonstrated that hormonal status contributes significantly to higher mortality, explaining why men have a higher mortality rate and disease severity than women [3]. Men with COVID-19 are also more likely than women to develop complications (58% versus 42%). According to the literature, men with mild testicular infections face potential risks of infertility [4].
It has been discovered that SARS-CoV-2 affects the concentration and motility of sperm, thereby destroying male fertility [5].
However, the mechanism of infecting male and female tissues by SARS-CoV-2 has not yet been determined. Nonetheless, it has been widely reported that spermatogonial, leydig, and sertoli cells express ACE2 [6]. This enzyme is also expressed in the proximal renal tubules, intestinal ducts, seminal vesicles, ciliated cells in the nasal mucosa, endothelial cells and pericytes in bronchi and fallopian tubes, epididymis, exocrine pancreas, gall bladder, and trophoblasts. The intensity and breadth of coronavirus gene expression can be related to the fact that mRNA functions independently without intermediaries and does not require RNA translation or editing.
This study investigated the genetic relationship between the coronavirus and the human genome using a comprehensive and straightforward method to uncover a quite complicated relationship. The BLAST tool of the NCBI-NIH database was utilized to analyze and evaluate the results of 24 FASTA sequences of SARS-CoV-2 from 21 different countries.
The 17-nucleotide sequence TCCTGCTGCAGATTTGG containing the PstI restriction site is the only sequence in the SARS-CoV-2 N gene identical to the human hg38 genome [7].
Likewise, this sequence was aligned with the GRCh38.p14 database of Homo sapiens using the NCBI-NIH BLASTN tool. This 17-nucleotide sequence has significant and identical alignments with the five human chromosomes 7, 8, 10, 3, and 12.
Significant alignments
They involve complete alignment, respectively, with SHH, RNF32, EPPK1, and SLIT1 gene sequences, as well as the 16-nucleotide alignment with TNIK, PLD1, SLITRK3, PPP2R2B, EBF2, UBE2V2, EFCAB1, PTHLH, CCDC91, and PZP sequences, with the following alignment positions (Figures 1-5).
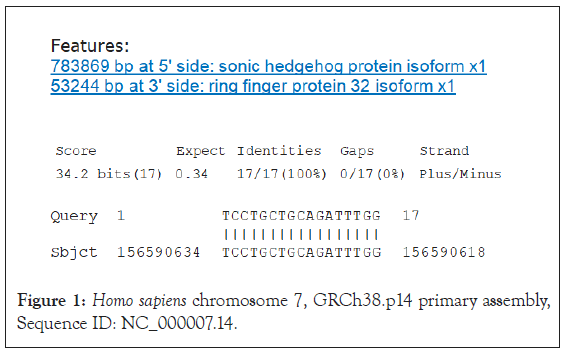
Figure 1: Homo sapiens chromosome 7, GRCh38.p14 primary assembly, Sequence ID: NC_000007.14.
Figure 2: Homo sapiens chromosome 8, GRCh38.p14 primary assembly , sequence ID: NC_000008.11.
Figure 3: Homo sapiens chromosome 10, GRCh38.p14 primary assembly, sequence ID: NC_000010.11.
Figure 4: Homo sapiens chromosome 3, GRCh38.p14 primary assembly, sequence ID: NC_000003.12.
Figure 5: Homo sapiens chromosome 12, GRCh38.p14 primary assembly, Sequence ID: NC_000012.12.
Function of genes
There seems to be a PstI-mediated co-colonization relationship between coronaviruses and their human homologs. This relationship can be established through short and long genetic similarities shared by all organisms. This 17-nucleotide sequence containing the PstI restriction enzyme facilitates the selective generation of hybrid DNA molecules and is thus useful for DNA replication and cloning. Horizontal gene transfer from a common ancestor is likely responsible for restriction enzymes' evolution, dispersal, and ubiquity. By these seventeen common unique nucleotides, the complex of the coronavirus, PstI, and the specific genes mentioned in humans likely issue a new command genetic code that acts against the human system. This may be due to the dysfunction of the genes or proteins mentioned, ultimately promoting the growth and development of the coronavirus. The following section discusses the function of the listed co-aligned genes, whose description and causal interpretation serve as a selfcertification and signature of the connection of these genes with the coronavirus and the disease symptoms, as well as proof of the potential use of smart reverse engineering by the coronavirus to utilize these genes:
RNF32: This gene has extremely high protein and RNA expression in male tissues, especially in the testis and epididymis. It also has low gene expression in female tissues, the brain, the eye, endocrine, and gastrointestinal tissues. RNF32 has the highest level of immune cell-type RNA expression in basophils. Recent evidence indicates that basophils are a significant source of the cytokine interleukin-4, possibly more than T lymphocytes. Interleukin 4 is an essential cytokine in developing allergies and producing IgE antibodies by the immune system. This gene may also be a possible contributor to the coronavirus-induced cytokine storm mechanism. The gene's protein function may play a key role in sperm formation, which requires protein-DNA or protein-protein interactions. This gene is most likely expressed in spermatocytes or spermatids during spermatogenesis, and its alternative splicing generates multiple transcript variants.
SHH: This gene expresses high levels of RNA in the kidney, urinary bladder, female tissues, male tissues, liver, and gallbladder. The SHH gene is one of the most significant endoplasmic reticulumactive genes among these aligned genes. The nsp6 protein is involved in the initial induction of host endoplasmic reticulum autophagosomes, limiting the expansion of these phagosomes and facilitating the transfer of viral components to lysosomes. For the coronavirus to enter the host and transfer its components, the SHH gene or its protein probably interferes with the endoplasmic reticulum or the modified hACE2 receptor in the Endoplasmic Reticulum (ACE2-ER). These viruses do not need to enter the host cell nucleus for transcription since their Ribonucleic acid (RNA) can go straight to the ribosomes attached to the rough endoplasmic reticulum and make their antigens using the ribosomes of the host's living cells [8].
Strikingly, some of ER’s general properties are beneficial to viruses. For example, as the ER-to-cytosol retro-translocation machinery is an inherent apparatus in the ER, it represents an ideal conduit for certain viruses and bacterial toxins to enter the cytosol. Additionally, ER membrane’s ability to undergo constant budding reactions plays a crucial role during viral replication and assembly when viruses deform and rearrange the ER membrane to generate ER-derived structures used to support these processes. The membrane network of the host cell frequently supports virus replication and assembly. These membranes serve as scaffolds for viral and host components required for replication and assembly [9].
Moreover, this gene encodes a protein involved in early embryo patterning. Holoprosencephaly (HPE) is caused by a defect or malfunction in this protein or its signaling pathway. VACTERL syndrome symptoms, including vertebral defects, anal atresia, tracheoesophageal fistula, esophageal atresia, radial and renal dysplasia, cardiac abnormalities, and other anomalies, are thought to be caused by mutations in this gene or its signaling pathway.
Pathogen-mediated infections, including influenza-A and, more recently, SARS-CoV-2, have been linked to the SHH pathway. Recent evidence suggests that Shh signaling also contributes to inflammatory and immune responses in various diseases. Specific pathogens utilize this pathway to control the locally contaminated environment. The Shh pathway plays a role in human inflammatory diseases such as influenza-A infection, smoking-induced airway inflammation, arthritis, inflammatory bowel diseases, pancreatitis, colitis-related cancer, asthma, allergy, and atopic dermatitis [10]. Neutrophils and basophils express low levels of SHH RNA of the immune cell type.
The immune response to an invading virus may disrupt SHH signaling, leading to a cytokine storm characterized by multiple immune dysregulations, generalized symptoms, and multi-organ dysfunction. The cytokine storm is believed to be the primary mechanism underlying the devastating effects of the SARS-CoV-2 pandemic, also known as COVID-19 [10]. The virus can eventually cause tissue damage by activating the SHH signaling pathway.
Several viruses, including coronaviruses, utilize cellular autophagy to aid their replication. Acute respiratory syndrome coronavirus 2 mediates its replication via an ATG5-dependent or independent pathway employing double-membrane vesicles comparable to autophagosomes. Multiple mutations in NSP6, a non-structural protein with a positive regulatory effect on autophagosome formation, suggest the possibility of a relationship between SARSCoV- 2 pathogenesis and autophagy [11].
Viruses are obligatory intracellular parasites and must evade the host cell's innate immune defenses. Autophagy activation is emerging as a common response to viral infections. However, autophagy's effects on infection can swing from beneficial to detrimental, depending on the nature of the virus.
EPPK1: This gene exhibits high protein expression and low RNA expression in male and female tissues and in the kidney and urinary bladder. Furthermore, despite its low protein expression in the skin and brain, its RNA expression is high. This gene encodes a protein that contributes to the organization of the cytoskeleton and may preserve the integrity of keratin intermediate filament networks in epithelial cells.
SLIT1: This gene's RNA expression is low in male tissues, particularly in the testis, but high in brain and endocrine tissues. It is believed to function as a molecular guidance signal in cell migration. It also has low immune cell-type RNA expression in neutrophils and monocytes.
TNIK-TRAF2 and NCK interacting kinase: Wnt signaling plays a crucial role in carcinogenesis and embryo development. The serine/threonine kinase encoded by this gene activates the Wnt signaling pathway. The gene's mutation is associated with the autosomal recessive form of cognitive impairment. Alternative splicing generates multiple transcripts, encoding distinct isoforms. The gene exhibits high protein expression and low RNA expression in the bone marrow, lymphoid tissues, and male and female tissues but high RNA expression and low protein expression in the brain, eyes, proximal digestive tract, and muscle tissues.
PLD1: This gene exhibits extremely high protein expression and moderately high RNA expression in female, male, endocrine, and gastrointestinal tissues. It is highly expressed in basophils but has a lower level of expression in eosinophils, neutrophils, and monocyte. It encodes a phospholipase specific to phosphatidylcholine. This enzyme may be involved in intracellular trafficking and signal transduction. Alternative splicing generates multiple transcripts with catalytic and regulatory properties.
SLITRK3: This gene is highly expressed in female and male tissues, the liver, the gallbladder, and the brain. It encodes a member of the SLITRK family of structurally related membrane proteins involved in neurite outgrowth regulation. Alternative splicing generates multiple transcript variants encoding the same protein. This gene is overexpressed in numerous types of tumors.
PPP2R2B: This gene exhibits high protein and low gene expression in male and female tissues and brain tissue. Importantly, PPP2R2B interacted with PPP1R15A in the ERK signaling pathway, which is on chr9 and was associated with SARS-CoV-2 infection in the C5 phenotype from HGI (COVID-19 Host Genetics Initiative group) [12]. Two COVID-19 susceptibility loci were identified for multitrait testing in the EUR group.
EBF2: The protein encoded by this gene is a non-essential helixloop- helix transcription factor of the COE family. COE family proteins play important roles in a variety of developmental processes. TF binding sites of the EBF2 gene exclusively exist in the human-isolated SARS-CoV-2 genome near the start site of each viral gene or ORF (TF name: EBF2-GTCCCTTGGGATA) [13].
UBE2V2: This gene is highly expressed in female and male tissues, the liver, and the gallbladder. It encodes a protein similar to the ubiquitin E2 type 1 conjugating enzyme and the yeast MMS2 gene product. It may be involved in monocyte and enterocyte differentiation. As a Papain-Like Protease (PLPro) is a target shared by all coronaviruses. PLpro cleaves a portion of the viral replicase polyproteins into essential non-structural protein subunits required for the viral replication cycle. PLpro can additionally sequester ubiquitin and the ubiquitin-like protein ISG15 from host cell substrates to evade innate immune responses during infection [14].
EFCAB1: Extremely high protein and gene expression levels have been found in female and male tissues and the respiratory system.
PTHLH: This gene is responsible for most malignant humoral hypercalcemia instances. Mutations in this gene are linked to Brachydactyly type E2 (BDE2). There is also evidence for alternative translation initiation from non-AUG start sites, namely CUG and GUG, downstream of the AUG initiation codon, resulting in nuclear forms of this hormone. Despite high gene expression, protein expression is low in female and male tissues.
CCDC91: It regulates membrane traffic within the Trans-Golgi Network (TGN) and collaborates closely with GGAs to sort hydrolases into lysosomes. Its biological process involves the transport of proteins. Extremely high levels of protein expression and gene expression are found in both female and male tissues and in most organs.
PZP: It exhibits a high protein expression in male tissues, the liver, and the gallbladder. Gestational protein plays a role in pregnancy-related biological processes and tissue protection. It was one of the proteins significantly dysregulated in COVID-19- recovered cases. Patient recovery was correlated with immune response dysregulation. This broad-spectrum immunosuppressive protein inhibits the T-cell function during pregnancy to prevent fetal rejection; its overexpression is related to airway infection and bronchiectasis disease severity. The PZP serum protein level can be a biomarker for COVID-19 disease symptoms and the prognosis of asymptomatic carriers [15].
All aligned genes exhibit variable gene and protein expression levels in male and female tissues, consistent with their roles and responsibilities. This highlights the correlation between the coronavirus and the aligned genes via the distinct sequence of 17 nucleotides and its association with dysfunction and diseases of male and female tissues. These findings can be used in further research, clinical genetic testing, and patient data analysis. Like human evolution, other organisms, including viruses, seem to evolve and progress genetically. The biological systems of humans and other organisms are managed by their genetics. Accordingly, we should seek to discover their genetic brains, i.e., a genome sequence portion responsible for biological system management, evolution, and natural improvement in eukaryotes and prokaryotes.
According to the results of this study, similar to the short communications between people, such as daily verbal and emotional interactions in public and private spaces, which can be complex with significant outcomes, the short and small genetic connection between microbes and human genes can also have significant consequences. The genome is an intelligent system. The synapses established by the identical short sequences shared by the virus and human genes may activate or inactivate specific cell signaling pathways, triggering the development of pathologies in human tissues and organs. Connection, transmission, or genetic migration may play a significant role in abnormal growth or aging and the demise of organisms. Despite the identification of numerous types of gene transfer, we know considerably less about intelligent gene transfer. Using bioinformatics tools, researchers can better understand the interplay between molecular, genetic, biochemical, and biophysical mechanisms, improving their ability to pinpoint disease origins and inform therapeutic decisions.
Thanks to the creator of the universe. Thanks to the NCBI Database for the free sharing of coronavirus data used in this study. Thanks to the Human Protein Atlas proteinatlas.org for sharing the reference information of human genes.
Citation: Babaee H (2023) Correlation of SARS-CoV-2 Infection with the Disease Model and Reproductive Tissue Dysfunction as Signed by Human Genes. Advac Genet Eng.12:215.
Received: 10-Apr-2023, Manuscript No. MAGE-23-23382; Editor assigned: 13-Apr-2023, Pre QC No. MAGE-23-23382 (PQ); Reviewed: 27-Apr-2023, QC No. MAGE-23-23382; Revised: 04-May-2023, Manuscript No. MAGE-23-23382 (R); Published: 11-May-2023 , DOI: 10.35248/2169- 0111.23.12.215
Copyright: © 2023 Babaee H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.