Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2024)Volume 13, Issue 5
Background: Sleep deprivation has been associated with a variety of pathological conditions. Cortisol demonstrates a robust circadian rhythm and has been implicated as a synchronizing hormone for peripheral tissues. Cortisol will increase in response to high-intensity exercise and may be a useful treatment for circadian misalignment caused by sleep deprivation. However, it is currently unknown if cortisol responses to exercise would be affected by sleep deprivation. Therefore, the purpose of this study was to investigate whether cortisol responses to exercise performed following 24 hours of sleep deprivation would differ from responses following regular sleep.
Methods: Participants (n=8) completed a high-intensity cycling protocol following both sleep and sleep deprivation. Serum samples were analyzed to assess cortisol and glucose concentrations before and after the high intensity exercise. Biomarker data were analyzed via two-way repeated measures ANOVA with Bonferroni post-hoc analysis.
Results: Serum cortisol concentrations increased following the cessation of exercise, before falling toward baseline levels. There was no significant interaction effect between condition and time (p=0.969) or main effect of condition (p=0.262).
Conclusion: These results demonstrate that short duration, high-intensity exercise induces a significant increase in cortisol concentration, regardless of sleep status.
Sleep-deprivation; Endocrinology; High intensity exercise; Circadian
Sleep is considered restorative and essential for the health and wellbeing of humans. Sleep restriction is defined as the partial loss of sleep whereas sleep deprivation refers to the absence of sleep. Both sleep restriction and sleep deprivation are common in society and can occur both acutely and chronically. The health consequences associated with sleep restriction can be severe, whether the result of environmental issues (e.g. shift work) or due to sleep disorders (e.g. insomnia). Sleep restriction and sleep deprivation have been implicated in numerous pathological states, including cancer, diabetes and cardiometabolic risk factors. These sleep-related metabolic conditions have been suggested to be associated with disruptions within the endocrine system [1]. Cortisol is the final product in the hypothalamic-pituitary-adrenal axis and is largely considered an endocrine marker of stress. Cortisol follows a circadian rhythm, peaking in the early morning soon after waking and reaching its nadir in the evening. Sleep deprivation acts as a homeostatic disturbance, resulting in changes in the diurnal pattern of cortisol secretion and an increased cortisol concentration across a given time period. In addition to the potential diurnal shift, a stress induced cortisol secretion may be superimposed on the regular circadian rhythm through psychological and metabolic stressors, such as forcing oneself to stay awake during regular sleep periods or participating in physically demanding tasks such as exercise. Cortisol concentrations will typically increase with exercise, provided intensity thresholds are met and has pleiotropic effects during and following exercise, including increasing lipolysis and gluconeogenesis which serve to increase substrate availability.
The cumulative stress on the endocrine system is greater following sleep deprivation and thus the HPA-axis may demonstrate a lower reactivity to exercise when sleep deprived [2].
The role of cortisol as a master circadian synchronizing hormone has recently been discussed. In short, the ubiquity of glucocorticoid receptors in almost all human cells (and notably absent in the suprachiasmatic nucleus), the large proportion of the human genome under circadian and glucocorticoid regulation, the entrainment of the HPA-axis to external diurnal stimuli (i.e. light) and the interaction between glucocorticoids and intracellular clock proteins all suggest that cortisol has physiological relevance that extends well beyond a stressresponse hormone [3].
Sleep deprivation has been demonstrated to affect peripheral clock regulation. Peripheral clock regulation has been suggested to be modifiable by exercise and animal research has demonstrated a role for exercise-induced glucocorticoid increases to phase-shift circadian clocks. Taken together, this suggests that the cortisol response to exercise, if performed at the appropriate time, may serve as a re-alignment treatment for peripheral clock proteins in individuals that are sleep deprived. This may in turn serve as a therapeutic or preventive intervention to treat circadian rhythm related disorders [4].
Martin, et al. demonstrated elevated cortisol concentrations in response to higher intensity exercise relative to low-intensity exercise, although no difference was observed when comparing sleep deprived and non-deprived conditions. Other researchers have suggested that cortisol responses to exhaustive endurance exercise are similar following sleep restriction, but differences are observed after 30 minutes of recovery. Shorter duration and higher intensity exercise interventions are increasing in popularity due to the multitude of metabolic benefits. However, it is currently unclear whether the cortisol responses to this style of exercise would be augmented following sleep deprivation or whether differences would be observed during the recovery period. Therefore, the overall purpose of this study was to assess the cortisol responses to sleep deprivation and to assess hormonal changes in response to high intensity exercise and in the subsequent recovery period [5].
Ten male participants (20.6 ± 1.4 yrs, 26.6 ± 2.5 kg.m-2, 20.9 ± 6.8%) completed this study. All participants provided written informed consent approved by the institutional review board prior to participation. Participants were screened for participation as previously reported and excluded if body fat was >30%, were currently restricting calories or taking medications to aid weight loss or self-reported a history of endocrinological or metabolic disorders. Participants were also required to be non-smokers, have no documented sleep disorders or selfreported disturbed sleep patterns and completed no transmeridian travel within a month of testing. Lastly, participants were excluded if they engaged in planned exercise for >10 hours per week or had participated in sprint-style training within the previous 6 months. Following the informed consent process, participants were familiarized with an electronically-braked cycle ergometer and open-circuit spirometry system that would be used during the study [6].
A counter-balanced design was implemented to observe acute responses to exercise following a night of regular sleep and following 24 hours of complete sleep deprivation. The order of the two testing conditions were assigned at random. Each testing day required participants to refrain from activity for 48 hours prior to their scheduled visit and were separated by at least 3 weeks. In both conditions, participants reported to the laboratory at 0800 h and the environmental temperature and light exposure were maintained until 2200 h (the sleep deprivation condition maintained ambient conditions throughout the entire visit duration). During the sleep condition, participants were permitted to sleep between 2200 h and 0600 h; if participants were unable to remain asleep, they were asked to remain in bed in complete darkness until 0600 h. During sleep deprivation, participants were permitted only to perform resting level activities, such as reading or consuming media. Research assistants monitored participants constantly throughout this period to ensure compliance [7].
For both sleep and sleep deprivation conditions, an intravenous catheter was placed at 0600 h. Resting expired gases were then collected for 25 minutes via open-circuit spirometry. Participants then completed a 5-minute standardized warm-up (4 mins at 60 watts, 30 seconds at 80 watts and 30 seconds at 100 watts) on the cycle ergometer prior to a high-intensity sprint-style exercise protocol. At approximately 0700 h, participants completed 4 maximal exertion sprints on the cycle ergometer, in which the electronic resistance was set to 7.5% of the participants’ body mass (kg). Each sprint was followed by 4 minutes of active recovery at 50W, so that the total exercise time was 18 minutes. The exercise responses have been reported elsewhere [8].
Blood was collected by a trained technician via a catheter in the antecubital vein. For cortisol analysis, blood was collected at 8 time points: 60 mins prior to warm-up (time 1), 45-minutes prior to warm-up (time 2), at the onset of exercise (time 3) and 20, 45, 60, 75 and 90-minutes post-exercise onset (time 4-8). Additional blood samples were collected 30 minutes prior to warm up and immediately post exercise for determination of blood glucose. Samples were permitted to clot at room temperature prior to being centrifuged at 3000 rpm for 15 minutes at 4°C. Serum was aliquoted and stored at -80°C until analysis [9].
Biochemistry
Cortisol was analyzed via standard ELISA techniques using commercially available kits (R and D systems, Minneapolis, MN, USA, intra-assay CV, Inter-assay CV:). Intra-assay and inter-assay Coefficients of Variation (CV) were 8.2% and 11.2% respectively. Blood glucose concentration was analyzed via colorimetric assay (Cayman, Ann Arbor, MI, USA; intra-assay CV: 4.5%, inter-assay CV: 20.8%).
Statistical analysis
Two-way repeated measures Analysis of Variance (ANOVA) was used to model the main effect of time (8 time points), the main effect of condition (sleep vs. sleep deprivation) and their interaction.
Two-way repeated measures ANOVA was also used to model the change in blood glucose from pre-exercise to post-exercise and the effect of sleep or sleep deprivation. An alpha level of p<0.05 was used for all statistical tests. For significant main effects, post-hoc analysis with Bonferroni correction was completed. All statistical analyses were completed in the statistical software JASP [10].
Missing samples resulted in participant exclusion from final analysis. Eight participants had samples at all time points and were subsequently included in analyses. Blood glucose concentration increased from pre to post-exercise (F(1,7)=15.23, η2=0.69, p=0.006), but demonstrated no significant interaction with sleep condition (F(1,7)=1.12, η2=0.13, p=0.347) (Figure 1) [11].
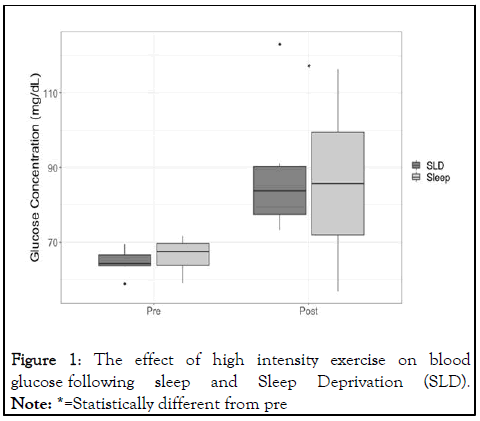
Figure 1: The effect of high intensity exercise on blood glucose following sleep and Sleep Deprivation (SLD).
Note: *=Statistically different from pre
Results from the two-way repeated measures ANOVA model demonstrated a significant main effect of time (F(7,49)=28.54, η2=0.80, p<0.001), but no interaction effect (F(7,49)=0.253, η2=0.04, p=0.969) or main effect for condition (F(1,7)=1.49, η2=0.18, p=0.262). Post-hoc analysis demonstrated that cortisol concentration was stable between the two pre-exercise timepoints (p>0.05) and immediately after the onset of exercise (p>0.05).
In contrast, circulating cortisol concentrations increased 20, 45, 60 and 70 minutes after the onset of exercise relative to -60 mins (all p<0.01), -45 mins (all p<0.01) and 0 mins (all p<0.01). Cortisol concentrations were still elevated at 90 mins after exercise onset relative to 0 mins (p=0.037) but were not different from -60 mins (p=0.108) or -45 mins (p=0.381) (Figure 2) [12].
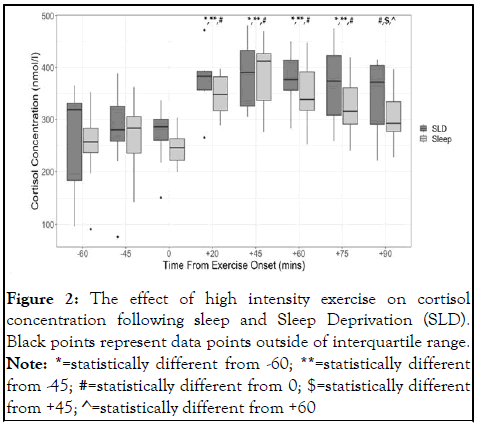
Figure 2: The effect of high intensity exercise on cortisol concentration following sleep and Sleep Deprivation (SLD). Black points represent data points outside of interquartile range.
Note: *=statistically different from -60; **=statistically different from -45; #=statistically different from 0; $=statistically different from +45; ^=statistically different from +60
This study was designed to extend our understanding of the combined effects of sleep deprivation and exercise on hormonal responses. We compared responses to high-intensity exercise following both sleep deprivation and regular sleeping conditions. The results indicate that cortisol concentration increases with high-intensity sprint style exercise similarly after a night of regular sleep or after sleep deprivation.
The increase in cortisol concentration following high-intensity exercise is congruent with the well-known exercise intensity threshold. Cortisol has been demonstrated to increase in response to a single cycling sprint at a resistance of 7.5% of body mass-as employed in the present study-and continue to increase for 20 minutes following the effort. Likewise, two repetitions of these sprinting efforts, separated by 4 minutes of recovery, have been demonstrated to increase cortisol concentrations by 45%. The four repeated efforts completed by the participants in the present study represents a substantially greater total work output and thus the robust stress-response observed is expected and reasonable [13].
With regard to sleep deprivation, the present results are in partial agreement with the findings of previous studies observing the cortisol response to exercise following disrupted sleep. Abedelmalek, et al. demonstrated no effect of sleep restriction (4.5 hours) on cortisol responses to two maximal exertion running sprints with 15 minutes of recovery between the bouts. Contrary to the robust cortisol response to exercise in the current study where participants maximally exercised for a short duration, Abedelmalek, et al. reported no change in cortisol concentrations in response to repeated running efforts at 80% of maximal sprint speed. Since the pattern of cortisol responses were similar between sleep conditions in our study, it is possible that the disparity with the running protocol was due to the exercise load and not in the use of complete, as opposed to only partial, sleep deprivation.
Further, the lack of detected difference in cortisol concentrations in response to the exercise stimulus may be due to the timing of blood samples (i.e. 60 minutes post exercise) as compared to the more frequent sampling in the current study.
In contrast to the observations by Mougin, et al., our results show no differences between the sleep and sleep deprivation conditions with respect to the cortisol concentrations post-exercise. This may be a result of the similarity in blood glucose concentrations regardless of sleep condition, at both rest and in response to the exercise challenge. This implies the metabolic signaling for increased HPA-axis activity and subsequent cortisol concentrations was similar between sleep conditions. Moreover, the exercise challenge employed in the present study may have been robust enough to mask any circadian HPA-axis disruptions due to sleep deprivation and thus similar exercise responses were observed.
Typically, the HPA-axis and Autonomic Nervous System (ANS) demonstrate an inverse coupling, such that high parasympathetic tone is associated with lower circulating cortisol and vice versa. In the present study, resting heart rate and the maximal heart rate response to exercise were both significantly lower in the SLD condition compared to sleep (data not shown). When analyzed via the ANOVA model framework, cortisol was not significantly different between conditions; however, there was an observed trend towards increased concentrations following SLD, especially prior to exercise. This higher vagal tone and potentially higher cortisol concentrations suggest a decoupling of the typical HPA-axis/ANS relationship. Such a decoupling and loss of functional connectivity has been previously observed in disease states, but future research should investigate this phenomena in sleep deprivation directly, potentially with longer sleep restriction periods. It may also be possible that cardiovascular control is disturbed via sleep deprivation effects on myocardium membrane potentials, as opposed to affecting the ANS per se.
Cortisol demonstrated a clear increase in concentration in response to this high-intensity sprint cycling protocol, appearing to be greatest after the cessation of activity, regardless of the sleep status of the individual. Exercise-induced cortisol release could be used as a future adjunct therapy in aligning peripheral clock mechanisms. Since circadian cortisol concentrations peak 30-45 minutes post-awakening, aligning this short exercise bout with the typical awakening time would result in maximal cortisol concentrations occurring at approximately the same time of the regular circadian rhythm. Indeed, recent evidence has demonstrated the efficaciousness of exercise in realigning a circadian profile, although researchers also reported an interaction with an individual’s chronotype. The circadian variation in cortisol concentration is typically greatest in the morning period and thus the cortisol response to exercise tends to be lower when exercise is completed in the morning. Engaging in evening exercise and generating a peak cortisol concentration that is offset from the typical circadian concentration may be detrimental in conditions where circadian profiles are already disturbed. The chronopharmacology of exogenous glucocorticoid administration has been established for some time, but future research should consider the timing of endogenous cortisol stimuli (i.e., timing of exercise interventions) on health outcomes.
Despite these noteworthy findings, this study had several limitations to be addressed. First, there was a relatively small number of participants enrolled, possibly limiting our ability to detect differences between conditions. Second, the study was limited by the number of time points assessed. The measurement of cortisol concentrations following each sprint may provide insight into the minimum work required to stimulate cortisol release following sleep deprivation. In addition, collecting more samples during the post-exercise period (up to 24 hours later) may elucidate additional benefits or circadian disruption. Although no differences between sleep deprivation and regular sleep for cortisol were observed in response to exercise, it is possible that the metabolic effects of sleep deprivation do not manifest until a future time. Indeed, Labsy, et al. found cortisol to be greater in the evening following sleep deprivation. Thus, future studies should continue to sample for longer periods (i.e. 24-48 hours) to investigate not only the impact of sleep deprivation, but any residual benefit of exercise following sleep deprivation in restoring regular circadian rhythm.
We demonstrated a similar increase in cortisol concentrations following high-intensity exercise following 24 hours of sleep deprivation or regular sleep. In addition, a small mean effect was observed, with greater overall cortisol concentrations following sleep deprivation. Future studies should investigate the role of this exercise-induced cortisol peak on circadian misalignment and peripheral clock regulation.
The authors declare no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Anderson T, Ritsche K, Berry NT, Wideman L (2024) Cortisol Responses to High Intensity Exercise Following Sleep Deprivation. J Sleep Disord Ther. 13:540.
Received: 12-Mar-2020, Manuscript No. JSDT-24-3618; Editor assigned: 17-Mar-2020, Pre QC No. JSDT-24-3618 (PQ); Reviewed: 31-Mar-2020, QC No. JSDT-24-3618; Revised: 03-Jun-2024, Manuscript No. JSDT-24-3618 (R); Published: 30-Jun-2024 , DOI: 10.35248/2167-0277.24.13.540
Copyright: © 2024 Anderson T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.