
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Review Article - (2020)Volume 10, Issue 6
The current pandemic by COVID 19 leaves new teachings at every moment. One of them is that children (especially those from early childhood) have a viral load of COVID 19 up to 10 times higher than adults, even though they are, in their vast majority, asymptomatic. This is of enormous sanitary importance, since they are "healthy" carriers, who can transmit the disease. For this reason, the authors emphasize the need to treat this age group with nasal and oral carrageenan, in order to cut the chain of contagion.
Children; Covid-19; Symptoms
The early association identified between SARS-CoV with SARS-CoV- 2 was supported by the analyzes made to the protein S (spike) that characterizes these two viruses, where an important similarity in these transmembrane structures was made clear, making them practically superimposable each. The only significantly different portion is a furin-binding domain in the SARS-CoV-2 protein S, which could expand tropism or increase virus transmission, compared to SARS-CoV of 2003. On the other hand, one of the most conserved portions of the protein is the receptor-binding domain (RBD), which has a similar (or reportedly higher) affinity to angiotensin-converting enzyme type 2 (ACE2) in comparison with SARSCoV [1]. This functional receptor is found in tissues, including lung alveolar epithelium, arterial and venous endothelium, smooth muscle, renal tubular epithelium, and small intestine epithelium, largely explaining the clinical presentation of COVID-19 patients. Furthermore, it has been shown that the activation of the virus ACE2 binding also requires the participation of the TMPRSS2 receptors, the hyperexpression of which would justify the greater severity in men, especially those with androchronogenetic allopecia. Diagnostic confirmation is made through laboratory studies, which can be performed on a wide variety of biological samples.
In pediatric patients, fever and cough are the common symptoms of COVID-19 (Figure 1).
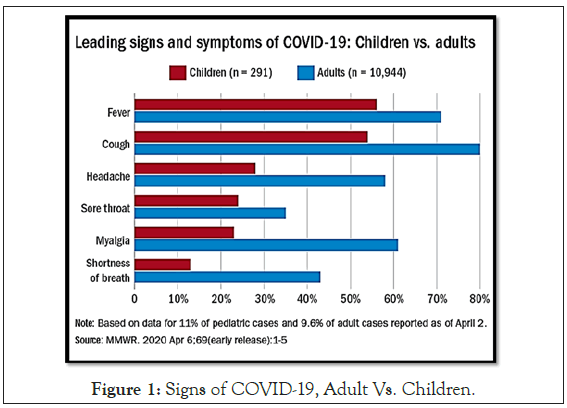
Figure 1: Signs of COVID-19, Adult Vs. Children.
Also odynophagia, excessive fatigue or diarrhea. In general, they will be mild, even asymptomatic (which does not prevent them from being contagious but, on the contrary, increases the risk of community spread).
Rates of serious illness were lower in older children, but there were rare cases of children in each age group requiring hospitalization. A small number of children between the ages of 2 and 15 have been observed to have experienced pediatric multisystemic inflammatory syndrome, or PIMS, for its acronym in English [2]. PIMS can cause vasculitis. PIMS have characteristics in common with toxic shock syndrome and Kawasaki disease. Children are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection but generally have mild symptoms compared to adults. Children cause the spread of respiratory and gastrointestinal diseases in the population, but data on children as sources of spread of SARS-CoV- 2 is scarce. Early reports found no strong evidence that children were the main contributors to the spread of SARS-CoV-2, but school closings early in responses to pandemic thwarted large-scale investigations of schools as a source of community transmission (Figure 2).
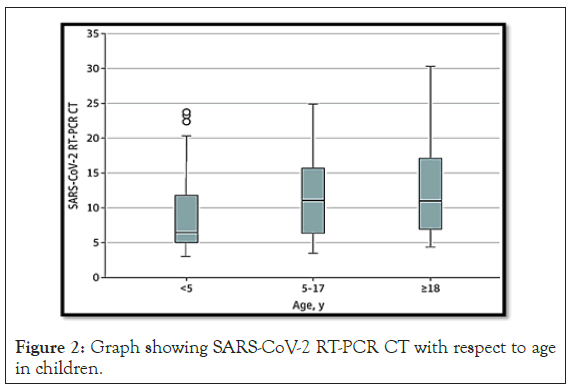
Figure 2: Graph showing SARS-CoV-2 RT-PCR CT with respect to age in children.
As public health systems seek to reopen schools and day care centers, understanding the potential for transmission in children will be important to guide public health measures. Replication of SARS-CoV-2 in older children leads to similar levels of viral nucleic acid in adults, but significantly higher amounts of viral nucleic acid are detected in children younger than 5 years. The behavioral habits of young children and locked rooms at school and daycare are of concern for the amplification of SARS-CoV-2 in this population as public health restrictions are eased. In addition to the public health implications, this population will be important in directing immunization efforts as SARS-CoV-2 vaccines become available [3].
An example of the risks that reopening (especially the school one) can cause if the appropriate measures are not taken, was Israel. At first, handling the pandemic in Israel was considered successful. It had closed schools in March and quickly implemented remote learning for its two million students. But after the face-to-face reopening, Jerusalem's Gymnasia Ha'ivrit High School soon became the largest single-school outbreak in the country and worldwide.
There were 154 students and 26 staff members infected. Despite the fact that the Ministry of Education had issued safety instructions such as the use of masks, the opening of windows, the frequent washing of hands and the safety distance, a key aspect failed: the social distancing [4]. It was thus that, inevitably, the coronavirus passed from schools to homes. And then to neighbors, who in turn transmitted it to other students, and it reached more educational institutes and teachers.
The Ministry of Education closed more than 240 centers and quarantined more than 22,520 teachers and students. The balance at the end of the school year was 977 infected students and teachers.
Many pointed to the anticipated reopening of schools as the trigger for a second wave of Covid-19 infections.
Siegal Sadetzki, who resigned as Israel's Director of Public Health Services, acknowledged that insufficient precautions in schools had to do with the new outbreak. Eli Waxman, a professor at the Weizmann Institute of Science and chairman of the team advising Israel's National Security Council on the pandemic, called the hasty reopening back to face-to-face classes a major failure. He also warned other countries not to follow their example [5].
Saliva contains crevicular fluid, desquamated oral epithelial cells, and microorganisms.
90% of saliva is secreted with a pH of 6 to 7.99% of saliva is water and the remaining 1% contains a large group of components. Saliva is known to play a role in early diagnosis and close contact transmission in COVID 19.
Chen et al. [6] found COVID-19 nucleic acid, suggesting that the salivary glands are infected by the new virus. Several studies have shown that the salivary gland and tongue express the ACE2 receptor, suggesting that the oral cavity is a perfect host for the invasion of COVID. In a previous study on severe acute respiratory syndrome-coronavirus (SARS-CoV), epithelial cells of the salivary glands were infected with high expression of ACE2. COVID-19 would generate infectious saliva in a sustained manner. ACE2 expression in the minor salivary glands was higher than in the lungs, suggesting that the salivary glands are a potential target for COVID-19. The positive rate of COVID-19 in the saliva of patients can reach 91.7%, and saliva samples can also cultivate the live virus. Some virus strains have been detected in saliva up to 29 days after infection with coronavirus [7].
Estimating the prevalence and contagion of new undocumented coronavirus infections is critical to understanding the overall prevalence and pandemic potential of this disease. Observations of reported infections in China, along with mobility data, a dynamic network metapopulation model, and Bayesian inference, have been attempted to infer critical epidemiological characteristics associated with SARS-CoV-2, including the fraction of undocumented infections and their contagion. An estimated 86% of all infections were undocumented (95% confidence interval (CI) 82%-90%) prior to the January 23, 2020 travel restrictions. The transmission rate of undocumented infections per person was 55% of the transmission rate of documented infections (95% CI: 46%-62%), however, due to their higher number, undocumented infections were the source of 79% of documented cases. These findings explain the rapid geographic spread of SARS-CoV-2 and indicate that containment of this virus will be particularly difficult. According to studies carried out on a larger scale, of 100% of infected people, 30% will not present any symptoms. This implies that it will not consult at any time, but it will be as infectious as manifest cases [8]. Del restante 70%, más de la mitad (cerca del 38%) se presentará oligosintomático, por lo que es dable que tampoco consulte (aunque la infectividad y contagiosidad estén presentes).
Of the remaining 70%, more than half (about 38%) will present oligosymptomatic, so it is possible that they also do not consult (although infectivity and contagiousness are present). As long as the tests are not really massive (not limited to multi-symptomatic patients), the true impact of COVID 19 on our society will be unknown.
In addition to restrictions on people's mobility, the World Health Organization and governments have prescribed maintaining an interpersonal distance of 1.5 or 2 m from one to another, to minimize the risk of contagion through the drops that we generally spread to around us by nose and mouth. However, recently published studies support the hypothesis of virus transmission at a distance of more than 2 m from an infected person (Figure 3).
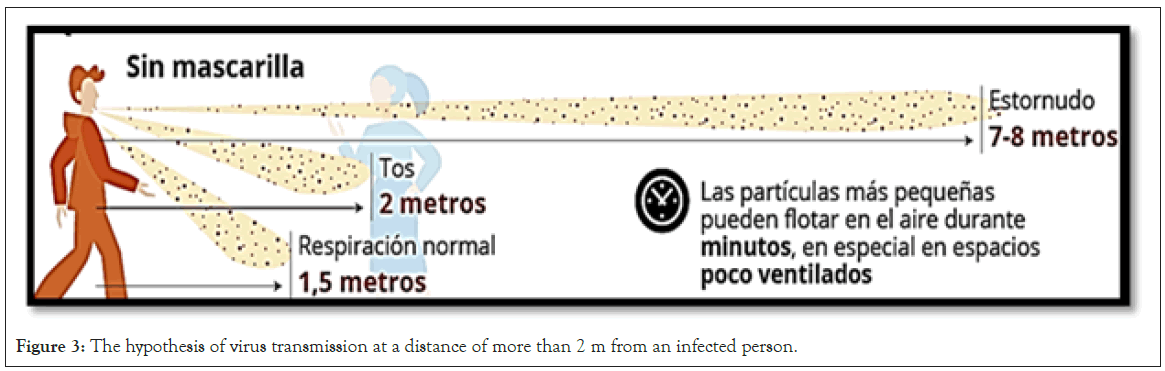
Figure 3: The hypothesis of virus transmission at a distance of more than 2 m from an infected person.
Researchers have shown the greater aerosol and surface stability of SARS-COV-2 compared to SARS-COV-1 (with the virus being viable and infectious in aerosol for hours) and that the transmission of SARS-CoV in air can occur at more than the anticipated distance between contacts. In fact, there is reasonable evidence about the possibility of airborne transmission of SARS-COV- 2 due to its persistence in aerosol droplets in a viable and infectious form. Based on available knowledge and epidemiological observations, it is plausible that small virus-containing particles can spread in indoor environments that cover distances of up to 10 m from emission sources, representing a type of transmission by aerosolization [9].
Field studies conducted within Wuhan Hospitals showed the presence of SARS-COV-2 RNA in air samples collected in hospitals and also in the surrounding area, leading to the conclusion that the air route should be considered a important pathway for viral spread. The interpersonal distance of 2 m can be considered reasonable as an effective protection only if everyone wears face masks in activities of daily living, but it is not sufficient in the case of aerosolization of secretions, and even less if external conditions (wind, etc. ) convey these secretions at a greater distance.
Current knowledge about carrageenans
Carrageenans are extracts from the Rhodophyceas seaweed.
There are 3 basic types of carrageenan: Kappa, Iota, and Lambda (Figure 4).
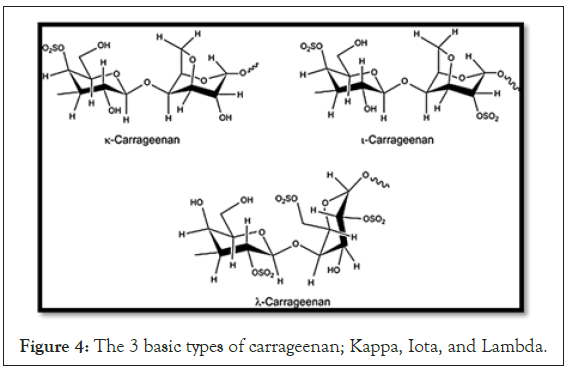
Figure 4: The 3 basic types of carrageenan; Kappa, Iota, and Lambda.
Carrageenans are used in the food industry either as a stabilizer, thickener or gelling agent. The foods most commonly treated with carrageenan are dairy products, meat products, pastry and confectionery.
Recently, the viricidal capacity of carrageenan has been reported, resulting from the interference with the early steps of viral replication, by inhibitory action on the viral coupling to the host cell. This effect is supposed to be mediated by the interaction of sulfated polysaccharides with positively charged domains on the glycoprotein envelope involved in binding with proteinglycans on the surface of the host cell. Thus, iota-carrageenan demonstrates a potent antiviral activity in vitro, reducing rhinovirus reproduction and its cytopathic effects. The same results were obtained with the herpes simplex virus and the Japanese encephalitis virus (Figure 5).
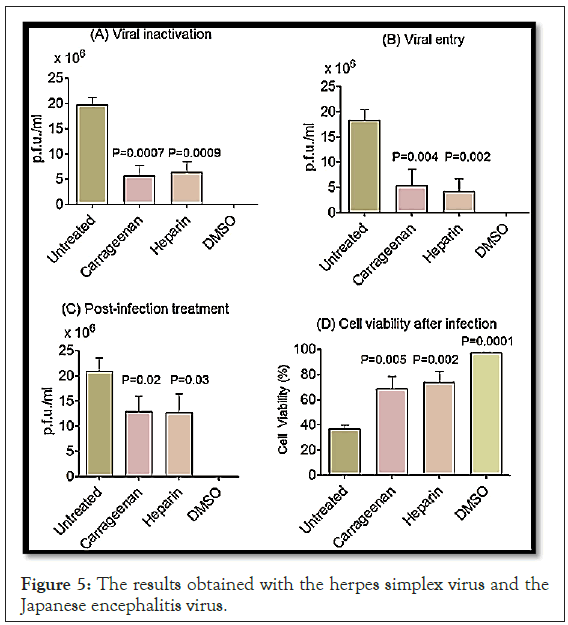
Figure 5: The results obtained with the herpes simplex virus and the Japanese encephalitis virus.
The binding and entry of coronaviruses, including SARS-CoV-2, is mediated by the Spike Glycoprotein (SGP).
Recently, a SARS-CoV-2 Spike Pseudotyped Lentivirus (SSPL) was developed that enables the study of spike-mediated cell entry through luciferase reporter activity in a BSL2 environment. Iota-carrageenan can inhibit cellular entry of SSPL in a dose-dependent manner. SSPL particles were effectively neutralized with an IC50 value of 2.6 μg/ml iota-carrageenan. In vitro data on iota-carrageenan against various rhino and coronaviruses showed similar IC50 values and was easily translated into clinical efficacy when a nasal spray containing iota-carrageenan demonstrated a reduction in the severity and duration of symptoms of the common cold, caused by various respiratory viruses. Consequently, our in vitro data on SSPL suggest that the administration of iota-carrageenan may be an effective and safe prophylaxis or treatment for SARS-CoV-2 infections. The antiviral action of carrageenan is due to the fact that this polymeric compound would function as an electrical barrier that, thanks to its negative charge, would bind to the viral particles, whose envelope contains positively charged proteins, thus preventing the virus from joining the virus. cell surface and blocking their entry into them. Carrageenan can also capture viral particles released by cells that have already been infected. Topical carrageenan has two different effects in relation to SARS-CoV-2. On one hand, it slows down infection through the nose in healthy individuals, by shielding the cells that form the epithelium of the nasopharyngeal mucosa. On the other hand, in the case of infected patients who were recently diagnosed, it prevents the viral particles released by the dying cells from colonizing new cells, for example from the olfactory epithelium, and that allows the pathogen to spread to new routes, to end up arriving to the central nervous system; or infect more cells of the respiratory epithelium, on the way to the lower respiratory tract (Figure 6).
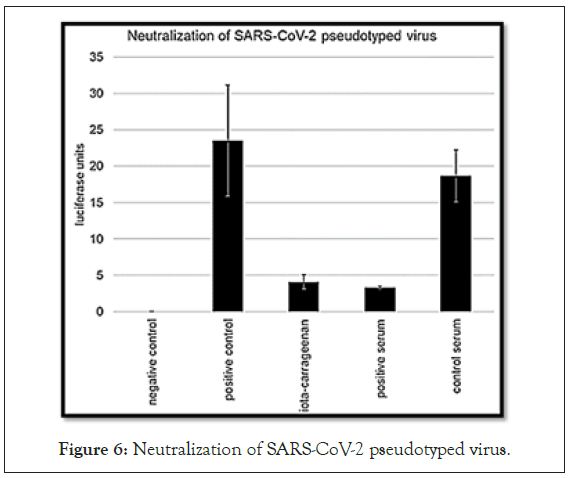
Figure 6: Neutralization of SARS-CoV-2 pseudotyped virus.
By preventing the virus from reaching the bronchi and lungs, the respiratory system would not be compromised, reducing the number of patients with COVID-19 in severe or even moderate condition [10].
Topical carrageenan is easy to apply; It has no side effects and gives a special resistance to entry, further proliferation and dissemination of the virus. It has been in the Argentine pharmacopoeia for almost 10 years, and in other countries (United Kingdom, Austria, Australia, etc.) for almost two decades. Carrageenan has come under fire, as some reports claim that it can cause potential damage to the digestive system. Meanwhile, the FDA along with the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) recognize carrageenan as a safe ingredient for consumption and a recent study further supports this idea. The debate over carrageenan is not new; It has been under careful investigation since the 1960s. Carrageenan is a key ingredient in foods and beverages, offering the desired stability, texture, and mouthfeel. Some even consider it to be the "perfect stabilizer." It is also offered as a vegetarian and vegan alternative to gelatin in confectionery products. The criticism surrounding the ingredient involves the fact that it is an "unhealthy" additive and that it is found in organic and natural products. Some health experts say it causes inflammation, and in severe cases it can lead to ulcers, bleeding, and even cancer. However, James McKim conducted a two-year study looking at the possible health outcomes of carrageenan. McKim's research confirms that carrageenan does not have an impact on the human body when consumed in food in the long term. Even less, when it is used as a medication, in the short and or medium term. In addition to antiviral active ingredients, typically, the present composition comprises at least one pharmaceutically acceptable carrier and, optionally, other additives or active ingredients. A suitable vehicle can be a diluent, for example water or saline, an excipient or other suitable and useful vehicle for the administration of the active ingredients. Carrageenan can be used in the form of any pharmaceutically acceptable salt, for example sodium salts of carrageenan can be used. Carrageenans of the iota type are available in the Argentine Pharmacopoeia (Nasitral). Carrageenan has been found to be non-toxic in oronasal administration, even at extremely high doses, and has therefore been classified as "Generally Recognized as Safe" (GRAS) by the Food and Drug Administration (FDA) from the US.
The antiviral pharmaceutical preparation is for the treatment or prophylaxis in an individual who is especially susceptible or at increased risk of a rhinovirus infection, such as a high-risk patient selected from the group consisting of an asthmatic patient, a person with allergies and a person suffering from an inflammatory disease (Figure 7).
Figure 7: The antiviral pharmaceutical preparation in the treatment or prophylaxis.
Typically, the composition will be provided as a sterile, nonpyrogenic preparation. However, the pharmaceutical composition could also come to be used to coat solid surfaces of hygiene or sanitary articles, for example, articles for hygiene or facial care that are typically used in the oral or nasal regions, such as handkerchiefs. or paper nose pads and pocket tissues [11].
More specifically, the pharmaceutical composition can be applied, for example, sprayed, in much the same way as disinfectants, onto toilet paper gloves, tissues or wipes, including nose tissues, to exert a vricidal effect, at least to some extent, thus helping to reduce repeated self-infection of an individual by contamination of the fingertips and also to reduce viral spread between different individuals who are in close contact with each other, for example, hand-to-hand.
Depending on the nature of the sanitary or hygiene article, said article may be covered, moistened or otherwise impregnated with the pharmaceutical composition [12].
Such carrageenan treated articles may also include, but are not limited to, cotton swabs, dust masks, or face masks. Even lipsticks can be formulated to contain an antiviral effective amount of iota-carrageenan. These hygiene or health care items can be used prophylactically or in conjunction with therapeutic treatment against a viral infection and can be helpful in preventing or reducing the risk of infection. Accordingly, the antiviral composition is applied by coating or impregnation on the solid surface of a hygiene or sanitary care article, in particular of a glove, washcloth or tissue of hygiene or sanitary paper, especially a tissue or paper towel for the nose.
If we consider the following facts:
• Drops and aerosols are a major source of human-to-human transmission.
• The sources mentioned above depend on different sizes of saliva droplets.
• The contagion comes from symptomatic and asymptomatic patients.
• The proportion of asymptomatic patients exceeds 90% of all pediatric cases.
• Carrageenan concentration is adequate in the nasal mucosa and salivary glands.
• Oral solution can offer a double protection: On the one hand, it reduces the spread and, on the other hand, it reduces the viral load.
• Carrageenan is in the pharmacopoeia, and its use is accepted almost worlwide.
• Its application does not imply any risk.
• We conclude that by using carrageenan in the form of a nasal and oral spray, we may be providing an inexpensive, safe and effective means of protecting people from contagion and serious forms of the disease.
Citation: Roberto RH, Héctor EC (2020) Covid 19: Children should be Treated Even in Absence of Symptoms. J Clin Toxicol. 10:457. DOI: 10.35248/2161- 0495.20.10.457
Received: 01-Oct-2020 Accepted: 15-Oct-2020 Published: 22-Oct-2020 , DOI: 10.35248/2161-0495.20.10.457
Copyright: © 2020 Roberto RH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.