Journal of Oceanography and Marine Research
Open Access
ISSN: 2572-3103
ISSN: 2572-3103
Research Article - (2017) Volume 5, Issue 2
In this study, the various floral species of two sites were surveyed for Macroalgal composition and floral zonation. No such study has been conducted for specific Ghanaian rocky shores such as Prampram and Takoradi, although more generalized studies are available concerning the coastal ecology of Ghana as a whole. Quadrat sampling was done to determine floral composition and distribution. Floral zonation was studied to investigate a discernable gradient in ecological health. The study focused on two similar rocky intertidal areas, 230 km apart, at Takoradi and Prampram, both in Ghana. Four belt transects were randomly laid from the lower to upper shores of each site and along which a continuous quadrat (1 m2) was placed to estimate species percentage cover (macroalgae). The species data was standardized before submitted to statistical analyses. Also two replicate water samples were taken at each site for the analyses of nutrients (i.e., nitrate and phosphate). Altogether a total of 36 species of macroalgae, comprising 9 Chlorophyta, 10 Phaeophyta, and 17 Rhodophyta were identified as present at both locations during the study. A multivariate analyses of the abundance and location of macroalgae in relationship to their level in the intertidal zone detected zonation differences in the macroalgal community structure between the western and eastern locations at Takoradi and Prampram respectively. It was observed that Rhodophyte species were more diverse in general than the other taxa at both sites. For Shannon-Wiener diversity, it is observed that samples for Takoradi are more diverse than Prampram. Species within algal taxa Rhodophyta are most abundant at both sites with Chlorophytes coming in next. The relative abundance for both Rhodophytes and Chlorophytes sampled from Prampram is higher than those from Takoradi. Phaeophytes at the other hand are higher in Takoradi than in Prampram. Polycavenosa dentata/ Hydropuntia rangiferina was the most abundant species occurring in the wet and dry periods. These observations provide a basis for future studies in determining conservation strategies for Prampram which is a fishing hub and Takoradi an industrialized city.
<Keywords: Macro-algae; Diversity; Rhodophyte; Phaeophyta; Chlorophyta
The importance of Macroalgae and their trophic link with intertidal fauna has been documented by numerous authors [1] the resultant being the proven relevance of these relationships in maintaining species richness and diversity within a community.
As such West Africa’s marine intertidal environment has been intensively studied in the past [2-4]. The study of intertidal zonation in Ghana was first documented by Bassindale [5] and Lawson [2,6] followed by several scientists including; Buchanan [7]; Gauld and Buchanan [8]; Edmunds [9]; John [10]; John and Lawson [11]; Biney [12] and Branoff et al. [13].
The tropical West African Flora is impoverished in contrast to the richness of the Caribbean region of the eastern Atlantic or the tropical coast of East Africa thus diversity is low. Like the western shores of other continents, coral reefs are absent and consequently so are the rich and varied life associated with these structures.
As a result of the absence of protecting coral reefs or shallow offshore shoals, much of the West African coast is very wave exposed [14]. Seasonal upwelling, seasonal inflow of turbid, silt laden water, seasonally lowered inshore salinity, absence of suitable shallow water substrata, low habitat diversity and heterogeneity are all factors contributing to the absence of coral reefs and the low species diversity of algae n tropical West Africa [14]. The works by these scientists gives prevalence to systematic temporal surveys of the intertidal environment as an aid in conservation purposes providing up to date baseline data.
Most of the previous researchers who worked on Ghana’s coast like Bassindale in 1952 and Lawson in 1956 [6], made an attempt to give a general picture of zonation on the entire coast of Ghana through an extensive survey. In reviewing their papers it was observed that macro algal diversity was almost similar across the whole length of Ghana’s coasts with abundances being different. This paper is intended to be an updated upshot and supplemental to the already proven facts by the above mentioned authors. However the focus of this paper is on two (2) selected sites (one in the East and the other in the West) on Ghana’s coast. This paper also outlines the prominence of periodic floral surveys in providing ecological managers with up to date baseline data of intertidal flora on Ghana’s coast.
Study areas
Located less than 50 km, east of Accra, Ghana’s capital, the Prampram Township is the largest community in the Dangme West District. As a coastal community, the primary occupations are fishing and trading in fish, but also there are farmers and artisans. The sampling site (5°42’17.68”N, 0°6’55.08”E) is chosen close to the old fort where artisanal canoes are moored as a result it being a moderately exposed beach with a berm of sand. This place also serves as a landing site for the fisher folk.
It is the third largest city in Ghana (4°52’41.78”N, 1°45’22.91”W). The capital of the Western Region, Sekondi-Takoradi, is an industrial and commercial centre of Western Ghana. The sampling site (4°52’41.78”N, 1°45’22.91”W) chosen is behind the Fisheries Commission office which offers a rocky beach with a wide sandy backshore beach. The beach slopes gently and is relatively level.
The area is moderately sheltered shore using Lawson’s shore type classification scheme which describes a more horizontal, rather than vertical layout of rocks along the shore with those closest to the sea taking most of the wave force and thus sheltering the rocks further in.
Field sampling
Sampling was done to investigate current diversity and zonation patterns of Macroalgae. The sampling design employed is the random stratified method of sampling. Sampling along vertical transects across the rocky shore. The method of sampling flora used was the point interception method [15] using a quadrat. At each site four belts transect was randomly laid from the lower to upper shores of each site and along which a continuous quadrat (1 m2) was placed to estimate species percentage cover (macroalgae). A vertical transect is a line along which data is collected that stretches from the high intertidal to the low intertidal. The beginning and end of our transect lines are typically marked by a bolt or marker embedded in the rocky reef. Vertical transects were chosen randomly and then coordinates fixed with a GPS with field sampling done at low tide. Tidal level predictions were determined using tide tables. This period offered opportunity for the widest possible area of the beach to be sampled.
The following physicochemical parameters were measured: dissolved oxygen, air and water temperature and nutrients (NO3, PO4). Air and water temperature were measured in situ using a multi parameter probe (Hannah multi parameter probe). Separate samples (3 replicates) were taken for dissolved oxygen and nutrients analysis. There were kept under ice and stored in thermos chest cooler and then transported immediately to the laboratory for analysis.
The percentage cover of Macroalgae was quantified using taxonomic guides and manuals [9,16].
Laboratory analysis
All flora species that were not identified on the field were stored in a refrigerator and using the appropriate guide [9,16,17] and compound microscope they were later identified. Dissolved oxygen and nutrients levels in the water samples were analysed following ISO protocols for NO3, SO4 and PO4 at the laboratory [18].
Data analysis
The species data was standardized before submitting to statistical analyses. The number of quadrat samplings depended on the beach width which in turn results from tidal levels and the geography of the area. The Shannon-Weiner, Margalef’s and Pielou indices for species diversity richness and evenness, respectively, were determined. The results ranges from zero to four; zero being void of both diversity and richness and four being extremely diverse and rich. The equations for Relative Abundance (RA), species diversity (H’) and species richness (d) indices are presented below.
Where n=The number of individuals in a sample from a population, ni=The number of individuals in a species (i) of a population, s=The number of species in a sample.
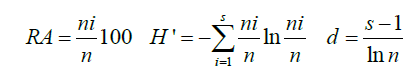
The results of this study highlight the distributions of individuals as well as groups of flora species within shore zones. Figures 1-3 present the distribution of floral species at Iture rocky beach for wet and dry seasons.
Species abundance of sampled macroalgae at sites in the eastern and western parts of Ghana’s coasts in the wet and dry seasons.
Jania rubens [19] accounts for 22% of the macroalgal individuals sampled at Takoradi and Hydropuntia rangiferina [20] accounting for 32% of macroalgal individuals sampled at Prampram. Generarally Cladophora species were mostly abundant in tidal pools. These were mostly at the upper portions of the beach. Ulva fasciata [21] was commonly found in tide pools in the upper and middle part of the shore where nutrients are high, wave forces are low and herbivory is reduced. Gelidium corneum occurred on rocky substrata, often on top of coralline crusts, normally associated with high levels of water movement, extending up to 1.5 m of intertidal elevation and down to 25 m deep, Ralfsia expansa, H. rangiferina [20] was generally found in shallow subtidal habitats but at times to 11 m depths. Centroceras clavulatum was found on rocks in middle to lower intertidal zones along strongly wave exposed shorelines. U. fasciata was the most abundant Chlorophyte species at both sites with relative abundance values of 275.8 {Takoradi} and 3016.5{Prampram} for wet period, 523.2{Takoradi} and 1170{Prampram} for dry period (Figures 4-9). For Rhodophytes J. rubens was the most abundant (1500 and 791) in Takoradi whilst H. rangiferina [20] was the most abundant Rhodophyte in Prampram with values 6012 and 1780 for wet and dry periods respectively. R. expansa [21] was the most the most abundant Phaeophyte at both sites with 196 and 240{Takoradi}, 150 and 216 {Prampram} (Figures 1-4).
Some notable species were found to express peculiar abundance at one site but occurs in lower degree or otherwise absent in the other. H. rangiferina/Polycavenosa dentata [20] occurred notably in the lower shore at Prampram in higher abundances (relative abundance; 1056) within the quadrat data than in Takoradi where it was far less or negligible. U. fasciata occurred at a higher relative abundance. It was well spread across shore whilst its relative abundance was lower in Takoradi and occurred in the midlittoral region. Bachelotia antillarium occurred only at Takoradi from the sampling data gathered. Bryopsis pennata also occurred at Prampram only, from the sampling data gathered. Caulerpa taxifolia and Enteromorpha flexiuosa had a higher abundance in Prampram from the sampling data (Figures 1-4).
Bachelotia antillarum, Caulerpa sp., Padina durvillei, and Polysiphonia ferulacea exhibited an increase in abundance in March on second time of sampling Takoradi. Sargassum vulgare also exhibited such a trend: Its abundance increased at Prampram in March. For both sites Ulva fasciata, P. dentata, Hypnea musiformes, Enteromorpha flexiosa, Colpolenea sp., Chaetomorpha linum species tend to increase in abundance in March. G. corneum and Lithothamnia sp. exhibited a decrease in abundance in March for both sites on second time of sampling. J. rubens found distinctly at Takoradi decreased in abundance on second sampling time (Figures 1-4).
G. corneum exhibited a clumped distribution at both sites. U. fasciata also exhibited a uniform distribution from the transect data at both sites. All other species exhibited a random distribution within quadrat data [22].
The was no significant difference in species diversity between the west and the east as postulated by Lawson [6]; Evans and Aguirre- Lipperheide [23] though the direction of the Guinea Current was suspected to affect species occurrences. As the two sites are different through hydrological and climatic factors as such difference in species is expected to be prevalent.
Comparatively Shannon-wiener diversity and margalef species richness for Rhodophyta was higher than Chlorophytes and Phaeophytes which means we sampled a lot of different species at notable abundances which belong to the taxonomic group Rhodophyta.
There was a higher level of dissolved nitrates in Prampram (0.75 mg/l) than in Takoradi (0.40 mg/l). This was perhaps due to high amount of human excreta which degrade at Prampram (Figures 9 and 10). This coupled with the low abundance of sea urchins may enhance the growth of Chlorophytes in Prampram. This was evident as C. linum and Enteromorpha flexuosa were better developed and covered a wider area in Prampram than in Takoradi (Plate A).
From the Bray Curtis dendrogram plot (Group average) for the two sites at 80% dis (similarity), samples taken for both wet and dry periods clustered together for their respective sites. Samples for Takoradi clustered at 70% similarity whilst those for Prampram clustered at 80 % similarity (Figure 11).
PCA plots showing the zonation patterns and the principal species characterizing such patterns
Vertical zonation and principal species at the eastern shore (Takoradi): Figures 12 and 13 are exemplified by: The principal species found in Takoradi are: Centroceras clavulatum, Corralina pilulifera, Jania Rubens, P. dentata, Lithithamnia biosporum, Padina durvilliae, R. expansa and U. fasciata (Figure 14). From the PCA (Figures 12 and 13) and SIMPER analysis done it is observed that: C. clavulatum is dominant mostly in the 11-20 m zone in Takoradi occurring least in the 1-10 m and 31-40 m, Corralina pilufera is most abundant in 31-40 m zone less or absent in the 1-10 m and 11-20 m. J. rubens starts from the 11-20 m zone and is much abundant in the 21-30 m still abundant in the 31-40 m. P. dentata occurs in high abundance in the 11-20 m and then recedes in abundance in the 21-30 m. L. biosporum occurs in the 31-40 m zone but starts from the 21-30 m. R. expansa has a moderately constant distribution from the 1-10 m zone to the 21-30 m zone. It is less abundant in the 31-40 m zone. U. fasciata was the principal Chlorophyte species; its abundance was highest in the 1-10 m, 11-20 m and 21-30 m zones but was lowest in the 31-40 m zones.
Differences in the zonation patterns during wet and dry periods: From the SIMPER analysis: Sampled Chlorophytes in November occurred in 11-20 m, 21-40 m and 31-40 m zones. In March they also occurred in the 11-20 m and 21-30 m zone also but had a lower average abundance than in November. They also occurred in the 1-10 m zone in March. Sampled Rhodophytes occurred in the all zones in November but they tended to occur only in the 21-30 m and 11-20 m zones in March with a lower average abundance.
Phaeophytes occurred in the 11-20 m zone for both periods but average abundance was higher in November.
PCA plots showing zonation patterns in Prampram and the principal component species that characterize such zonation
Vertical zonation and principal species at the eastern shore (Prampram): Figures 14 and 15 are explained as: The principal species from the PCA analysis which characterize the macroalgal community at Prampram are: C. clavulatum, G. corneum, P. dentata, R. expansa, E. flexuosa, C. linum, Hypnea musciformes, Bryopsis penata, S. vulgare, Boodlea composita, L. biosporum, C. taxifolia and U. fasciata.
C. clavulatum occurs in the 1-10 m zone and 11-20 m zone. G. corneum occurs abundantly in 31-40 m zone. Polycavenosa dantata occurs in abundance 10-20 m zone, 21-30 m zone and 31-40 m zone. However it is most abundant in the 21-30 m zone. R. expansa occurs dominantly in the 11-20 m zone but has significant distribution in the 21-30 m zone. It is absent in the 1-10 m zone and 31-40 m zone.
E. flexuosa occurs in the 1-10 m and 11-20 m zones. H. musciformes occurs highly in the 11-20 m and 21-30 m zones. It also occurs in the 1-10 m zone however at a much lower abundance. B. pennata occurs only in the 21-30 m in Prampram. S. vulgare occurs at a lower relative abundance in the 21-30 m zone and the 31-40 m zone. B. composita occurs only at low abundance in the 21-30 m zone. L. biosporum occurs predominantly in the 11-20 m and 21-30 m zones. It also occurs in the 31-40 m zones but at a lower abundance. U. fasciata only occurs in the supra-littoral 1-10 m zones.
Differences in the zonation patterns during wet and dry periods: Chlorophytes were more abundant in the 1-10 m zone in November whilst in March they were abundant in the 21-30 m zone. Rhodophytes occurred at 11-20 m, 21-30 m and the 1-10 m zone for both periods but average abundance was higher in the November. Phaeophytes occurred at 21-30 m for both periods but average abundance was higher in November.
45% of Chlorophytes sampled at Takoradi in the wet period of the year were found in the 11-20 m zone when sampling from the supra-tidal to sub-tidal along a transect. 60% Rhodophytes were found evenly in both 11-20 m to 31-40 m. All sampled Phaeophytes were found in the 11-20 m zone (Figure 16).
43% of all sampled Rhodophytes were found in the 11-20 m zone. 63% of all sampled Chlorophytes were found in the 1-10 m zone in the wet period of the year and 100% of all sampled Phaeophytes were found in the 21-30 m zone (Figure 17).
41% of Chlorophytes sampled in Takoradi were mostly sampled in the dry period where mostly found in 11-20 m zone sampling supratidal to subtidal along a transect. 90% Rhodophytes and Phaeophytes where mostly found in the 21-30 m zone (Figure 18).
38% of all Chlorophytes sampled in the dry period at Prampram were found in the 21-30 m zone. 33 and 31% of all sampled rhodophytes were found in the 11-20 m and 21-30 m respectively. 100% of sampled Phaeophytes were found in the 21-30 m zone (Figure 19).
This survey is meant to update existing data on rocky intertidal macroalgae at Prampram and Takoradi in Ghana. Also there isn’t any current periodic survey to capture temporal changes in the abundance and diversity of macroalgae in Ghana.
Far above the high water mark beyond the sandy back shore we encountered some xerophytes species notably Paspalum varginatum. Generally both sites have relatively flat rocks, with intermittent tidepools occurring in low depressions on the sites. There was presence of sea urchin (Echinometra spp.) As seen from the number of occurrence of sea urchin (Echinometra spp.) holes and at the uppermost elevations of the rocks there were no algae but presence of barnacles (Balanus spp.) In the habitats occupied by Balanus spp. it is often associated with sponges and encrusting red seaweeds on shady overhanging rocks and cave entrances and also bryozoans and ascidians in deeper shade [24]. The rock boring urchin feeds mostly at night from their burrows, consuming clumps of drift algae, or venturing out of the burrow to feed and then usually returning to the same whole [25-27]. In Panama, individuals were observed to clear the area around their burrows of all organisms except calcareous algae [28].
In the mid littoral portions of the intertidal shore we have rocks covered with a mixture of beautifully developed C. linum on the higher levels and just below E. flexuosa; in some areas we have Bachelotia antilarum. Chaetomorpha sp. is found capping the rock projections. Tide pools are dominated by P. dentata and Cladophora species with Littorina sp. covering the rock overhangs. Ulva flexiousa (Enteromorpha flexuosa) has a thinner parenchyma and is filamentous compared to U. fasciata hence U. fasciata dominates the upper littoral and Ulva flexiuosa dominates the lower littoral.
The Rhodophytes and Phaeophytes dominated the lower littoral. It was noted that in the west (Takoradi) U. fasciata was not very well developed compared to Prampram and this was due also to a greater abundance of sea urchins (Echinometra sp.) in Takoradi. Low abundance of sea urchins at Prampram is due to the community inhabitants (people) feeding on the Echinoderms.
Species composition and distribution
Floral composition: The floral species encountered represented Chlorophyta, Rhodophyta and Phaeophyta. These species were grouped under 3 phyla, 27 families and 36 species in general. For Takoradi an average of 34 species were encountered at sampling and 28 species for Prampram. Amongst the species observed, 32 of them were observed by Lawson and John [10], and John et al. [29]. They are notably; Asparagopsis taxiformis, B. antillarium [30], Bostrychia radicans [31], B. composita [32], B. pennata [33], C. taxifolia [34], C. clavulatum [35], Chaetomorpha antennina [36], C. linum [37]. Chnoospora minima, Chondracanthus acicularis [38], Cladophora sp. [39], Codium guineënse [40], Colpomenia sinuosa [41], Corrallina sp. [42], Cryptonemia crenulata [34], Cryptonemia seminervis [43], E. flexuosa [44], Enteromorpha prolifera, Galaxaura marginata [19], Gelidiopsis variabilis [45], G. corneum [46], J. rubens [47], Hydropuntia rangiferina/P. dentata [20], Hypnea cerviconus/Hypnea spinella [34], H. musiformes [46], Griffithsia schousboei [48], Lawrencia majuscula [49], Lithothamnia sp. [50], Lobophora variegata [51], Padina antillarum [52], P. durvillei [53], P. ferulacea/Neosiphonia ferulacea [54] R. expansa [22], S. vulgare [22], U. fasciata/Ulva lactuca [55].
General floral description for both sites
Upper shore: Floral species: Chlorophyte species such as C. linum [37], E. flexiuosa, U. fasciata [55] and Rhodophyte species such as G. corneum, were distinctively found in the upper shore. The Phaeophyte Bachelor antilirium [30] was also distinctly found at the upper shore.
Middle Shore: Chlorophyte species like U. fasciata was found at the middle shore (midlittoral) with a higher relative abundance compared to the upper shore. Rhodophyte species like G. corneum, H. rangiferina [20] occur at a higher relative abundance compared to the upper shore. C. clavulatum occurred in a high abundance distinctively only in the middle shore. J. rubens [47] occurs at the end of the middle shore and increases in abundance into the lower shore.
Lower shore: Lithothamnion sp., Jania rubens, Corolina sp. which are Rhodopyte species predominate the lower shore. Dictyopta celiolata, Padina durvillae, Lobophora sp., Gelidiopsis sp., G. marginata also occurred predominantly in the lower shore but at a less degree.
Ralfsia expansia occurs across shore but it tends to have a variable abundance which fluctuates across upper to the lower shore and this coincides with the increase in the bare rock exposure encountered during quadrat sampling.
Vertical zonation
From the illustration of biotic features on the vertical axes, showing the relationship of shore height and sea depth, with substratum (rock and grades of sediment), and the exposure of rocky habitats to wave action Costello and Emblow [56]. The study sites fall into an exposed or moderately sheltered rocky intertidal environment due to the following observations.
• Observed presence of Barnacles and mussels (Mytilus) in the eulittoral zone.
• Observed presence of Red Macroalgae and Corrallina in the eulittoral zone.
• Sea anemones, sponges & colonial ascidians in the infra littoral or sub-tidal zone of the rocky shore [56].
At both sites, we encountered some algal tuft or mats as well as lichens: which were rather a rare occurrence. Ephemeral green and red seaweeds in the supralittoral zone. In the eulittoral we encountered Barnacles and Corrolina sp. In the infralittoral we observed an abundance of sponges and anemones (Figure 20).
From the data gathered; Diversity trends (Figure 1) show that species richness and diversity is higher in Takoradi than Prampram because Prampram has a slightly stressed environment (Figures 11 and 12) with nitrates (NO3) being higher compared to Takoradi and high exposure to waves as such macroalgal species with narrow tolerance level to increased nitrates and wave exposure would have limited abundance giving way to the most adaptable to flourish competitively. Hence species richness and diversity would be low in Prampram compared to Takoradi. Chlorophytes are more diverse in Takoradi than Prampram, Margalefs richness higher and evenness lower compared to Prampram same trend is observed for Rhodophytes and Phaeophytes. For Shannon-Wiener diversity, it is observed that samples for Takoradi are more diverse than Prampram. Rhodophytes are most abundant at both sites with Chlorophytes coming in next. The relative abundance for both Rhodophytes and Chlorophytes sampled from Prampram is higher than those from Takoradi. Phaeophytes at the other hand are higher in Takoradi than in Prampram.
The principal component species in Takoradi are Centroceras clavulatum, C. pilulifera, Jania Rubens, P. dentata, L. biosporum, P. durvilliae, R. expansa and U. fasciata. With J. rubens being the most abundant species occurring in the wet and dry periods. The principal species in Prampram are: Centroceras clavulatum, G. corneum, Polycavenosa dentata, R. expansa, E. flexuosa, C. linum, H. musciformes, Bryopsis penata, S. vulgare, B. composita, L. biosporum, C. taxifolia and U. fasciata. With P. dentata/H. rangiferina being the most abundant species occurring in the wet and dry periods. It occurs in the 10-20 m zone, 21-30 m zone and 31-40 m zone. Some peculiar species from the sampling data occurred only at Takoradi these being J. rubens, P. ferulacea, and B. antillarium. Species structure remains almost constant in the same site for both wet and dry periods though it is distinct from one site to the other. Predation was most evident in Takoradi with the high abundance of sea urchin hole and presence of underdeveloped C. linum.
Zonation patterns change slightly in a site for November (Wet period) and March (Dry period) but average abundance is higher in November for all Major Macroalgal taxa due to increase in tidal range and shortening of the tidal cycle as viewed from the tide prediction tables. It is observed that Macroalgal communities in Prampram are characterized by Chlorophytes and Rhodophytes and Communities in Takoradi is characterized by Rhodophytes and Phaeophytes.