Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Review Article - (2020)Volume 10, Issue 1
For centuries, molecular hydrogen (H2) was supposed to be inactive and useless in the cells of mammals. Scholars reversed this notion by representing that H2 reacts with extremely reactive oxygen species (ROS) like hydroxyl radical (•OH) and peroxynitrite (ONOO-) in cells. H2 has appeared as a uniquely therapeutic and preventive agent, with its antioxidant, anti-inflammatory and anti-apoptotic properties confirmed in surplus of experimental animal models and human studies. H2 can be administered by different approaches, comprising direct inhalation in the form of hydrogen gas, oral intake of hydrogen-rich water (HRW), and injection with hydrogen-rich saline (HRS) against the majority of disease conditions. Numerous antioxidant systems are restricted and unsuccessfully picked up by organelles such as mitochondria, but H2 has the capacity to efficiently enter plasma membranes and penetrate into subcellular organelles, like nucleus and mitochondria. The therapeutic/protective capability of H2 is developing in several human infections and in their experimental animal models, comprising ischemia-reperfusion diseases, neurodegenerative diseases, cancer, graft-versus-host disease, multiple organ dysfunction syndromes, to mention some. The medical view of H2 offers evidence-based progress for beneficial use and prospect investigation on H2 for the extensive health of the society. This paper reviews current progress in protective and therapeutic uses of H2 against different human and experimental animal disease models.
Molecular hydrogen; Anti-oxidant; Anti-inflammatory; Anti-apoptotic; Disease models
Molecular Hydrogen (H2) is a non-metallic and the smallest atom made up of two protons and two electrons, which is colorless, odorless, tasteless, and non-toxic [1,2]. H2 is the lightest gas and the most abundant element in the space [3]. H2 gas is flammable and will scorch in the air at very varied ranges from 4% to 75% [2]. Its temperature of the spontaneous explosion in the air is approximately 500 °C [4-6]. Figures 1 and 2 recommend that H2 is not thus unsafe in everyday life activities as much as its amount is below 4% [4]. Even though, human beings and most mammals do not own cells that secret H2, an enormous amount of anaerobic microbes in the bowel could generate H2 by degrading herbal fibers and starches [7]. Medical use of H2 as a free radical scavenger was appeared in the 1970s [8]. The first impact of H2 in Science was reported by Dole and coworkers in 1975 [9]. Fascinatingly, in 1975, an investigation by Dole and coworkers described that hyperbaric hydrogen treatment exhibited a noticeable decline of tumors of squamous cell carcinoma in baldheaded albino mice [9,10]. It was also in October 1975 that a group of professionals from Texas A and M University and Baylor university reported freshly recognized healing properties of H2, previously thought as a biologically unreactive gas with little capability to react with many biomolecules [11].
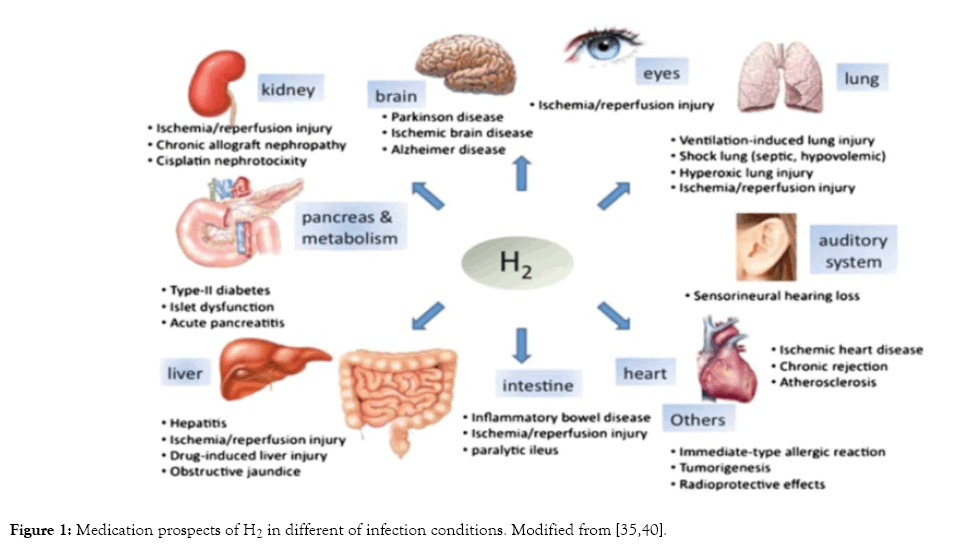
Figure 1: Medication prospects of H2 in different of infection conditions. Modified from [35,40].
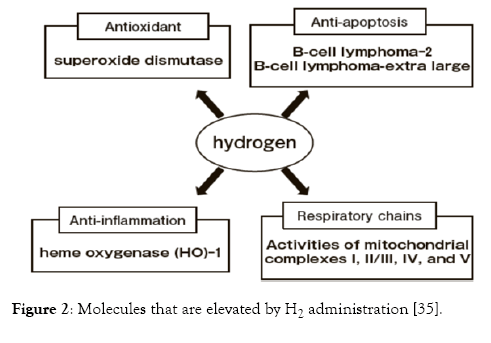
Figure 2: Molecules that are elevated by H2 administration [35].
According to contemporary surveys, H2 owns antioxidant, antiinflammatory, anti-apoptotic, anti-allergy and anti-cancer effects and could potentially apply to numerous cytoprotective roles [12-16]. Many researchers confirmed that H2 precisely and selectively scavenges extremely reactive hydroxyl radical (•OH) and peroxynitrite (ONOO-) but not hydrogen peroxide (H2O2), superoxide anion radical (O2-), or nitric oxide (NO), which are essential molecules for cell signaling [17-19]. Nowadays, a number of researchers have confirmed the preventive and therapeutic properties 2 in different organs, including the liver, brain, kidney, pancreas, lung and other chief structures, specifically in experimental animal models [20-22]. The curative ability of H2 has currently been reported in more than 170 various human and animal disease models [23]. H2 is hydrophobic, and the smallest possible molecule, therefore permitting it to rapidly spread via plasma membranes and reach the nucleus, mitochondria, endoplasmic reticulum, and other organelles or subdivisions of cells or tissues [23].
It was suggested that H2 could be not only a reserve for clean energy but also a hopeful resource medically [24]. So far, hundreds of research and review articles have already been published in this area in a number of diseases. Hence, this review summarizes the existing research outcomes and advances in therapeutic and protective uses of H2 in different animal and human disease models.
There are many ways by which H2 can be delivered into the body [17]. They are practically divided into the following major kinds: inhalation of hydrogen gas, ingestion hydrogen-rich water (HRW), and inoculation of hydrogen-rich saline (HRS) [17,25,26]. Each administration route has its own distinguishing features and benefits based on the nature of infection [27]. H2 could readily enter plasma membranes and arrive in internal cell parts [1].The majority of antioxidant counterparts are restricted in their cellular diffusions and are not easily taken up by subcellular compartments, but H2 has the capability to efficiently infiltrate plasma membranes and permeate into organelles, like nucleus and mitochondria [28,29]. In contrary to numerous other antioxidants, H2 has the benefit of being capable of infiltrating the blood-brain barrier [30]. Depending on the type of disease, the H2 administration differs [9].
Inhalation of H2 gas
H2 gas inhalation is a direct treatment technique. The inhalation of H2 gas could be done by giving H2 gas via a ventilator circuit, nasal cannula or facemask [28]. H2 gas inhalation can elevate the amount of H2 in venous and arterial blood vessels [31]. A medical investigation by Ono et al. indicated that inhalation of 3-4% H2 gas extended to the maximum level of nearly 10 μM and 20 μM respectively in the arterial and venous blood, in roughly 20 minutes affected no biological parameters, signifying the absence antagonistic properties [29]. Even though the wellbeing of breathed in H2 gas in humans is confirmed by its use in hydreliox, a striking breathing gas combination comprising 49% H2 utilized during extremely deep systematic diving [3], H2 inhalation treatment is quiet in the clinical trial steps of study[32]. To date, delivering H2 by inhalation is more of theoretical or not appropriate for continual intake for protective practices [32]. The therapeutic efficiencies of inhaled H2 were observed in the rat models of ischemia/reperfusion (I/R) damage to the lungs, small bowel and hemorrhagic shock [33]. Even though H2 causes little or no hazard of burning in air and oxygen when its level is below 4%, there is still danger in the production, packing, passage and utilization of hydrogen-oxygen mix [34].
Oral intake of HRW
Consumption of dissolved H2 in water is a convenient and easy means of application since it is safe and suitable [26]. H2 drenched water (1.6 ppm) is harmless and more useful than the breath in H2 gas [17]. The initial report in this field exhibited that free access to HRW protected impulsive progress of arteriosclerosis in Apo lipoprotein E knockout mice [17]. Drinking water holding curative quantities of HRW symbolizes a substitute model for the administration of H2 succeeding therapy of reactive oxygen species (ROS) generated disease conditions [35]. Consumption of water augmented with H2 denotes a unique technique of H2 gas supply that is certainly adaptable into clinical practice, with useful influence for numerous therapeutic situations, comprising atherosclerosis, metabolic disorder, type 2diabetes, and mental injury due to old age [36]. Moreover, oral intake of fluids containing H2 indicates an exceptional and harmless technique of administering H2 with HRW, displaying highly dissolved hydrogen, little dissolved oxygen, high pH and significantly negative redox potential values [37].Toxicological researches identified that a 60 kg human can drink HRW up to 1.2 L/day is safe [38]. HRW could be prepared using many techniques, comprising liquefying H2 into clean water by inducing a chemical reaction between metallic magnesium and water under high pressure [Mg+2 H2O → Mg (OH)2+ H2] [36,39].
Injection of HRS
Though giving oral HRW is suitable and safe, regulating the amount of H2 delivered might be hard, as it vaporizes in water and could be lost earlier than absorption [21]. H2 can be intraperitoneally or intravenously injected as HRS (H2 saturated in salt), which permits the transfer of H2 with unlimited efficiency in many animal models [29]. Delivering H2 through an injectable HRS system might permit the transfer of a more precise amount of H2 [40]. The mix may be prepared in a 1.6 ppm H2 volume. Delivering 500 ml intraperitoneally or intravenous HRS over 30 min for 3 days or more could alleviate the signs of fever and ache in patients with acute erythematous skin illnesses [29]. It has been described that HRS can protect or alleviate initial changes in disease conditions and guide to longterm practical progress in rat models of neonatal hypoxiaischemia [41]. Furthermore, it is an alternative to the therapy of influenza patients and other viral communicable infections. Hence, it could be most appropriate to select the injection of HRS method as the principal H2 treatment for serious viral transmittable infections [27].
The influence of H2 on different illnesses have been recognized to four main molecular bases: (1) a precise counteracting action of •OH, (2) mitigating the action of ONOO-, (3) signal modulating (4) modifications of gene expressions [2,42]. The initial mechanism recognized for H2 was its precise scavenging action of radicals. Undeniably, biomarkers of oxidative stress such as 8- Hydroxydeoguanosine (8-OHdG), (nucleic acid oxidation marker), 4-hydroxyl-2-nonenal (4-HNE), (a specific marker of lipid peroxidation) and malondialdehyde (MDA), are reduced in the entire surveyed rat models [43].
The other molecular mechanism of H2 influence is ONOOneutralizing action [2]. Though H2 couldn’t eradicate ONOOas effectively as •OH in vitro, H2 could capably decrease NO stimulated generation of nitrotyrosine [44,45]. Similarly, NO molecule exerts treatment properties, such as dilation of blood vessels and suppression of platelet accumulation [42,46,47]. These mechanisms are not mutually exclusive and some of them could be reasonably related to other mechanisms. Nevertheless, additional studies are essential to explain the detailed relations of the mechanisms [2]. Earlier studies were repeatedly concentrated on the first two mechanisms, and limited researches have been done on gene expression and signaling modulating pathway [48].
H2 could selectively decrease •OH and ONOO-, which are extremely aggressive ROS that extensively react with proteins, lipids, and nucleic acids, causing lipid peroxidation, protein denaturation and DNA breakup [49]. There is an accumulated body of the indication on the basis of animal model trials and clinical observations that H2 could be efficiently utilized as an antioxidant for the protection of oxidative damage-associated disease conditions [50]. It was identified that H2 can possibly decrease the amount of highly cytotoxic ROS (•OH), defending cells successfully [47,51]. Other researchers later confirmed that anti-oxidant properties of H2 on I/R damages of various organs like the heart, liver, and intestines [52]. In rat models of acute cerebral and coronary artery occlusion, inhalation 1% to 4% H2 alleviates the infarct dimension [53]. These innovative investigations were surveyed by publications from several sets globally indicating that 1.3% to 3% breathe in H2 prevents against critical oxidative damage [54]. Other researches have revealed that H2 has similarly a preventive property against I/R damage in the heart, liver, and intestine by suppressing oxidative stress [55]. Ingestion HRW protected prolonged anxiety brought injuries in learning and memory in mice by decreasing oxidative damage and defends nerve cells by motivating the ghrelin hormonal expression [56]. In present advancement in eye therapy, it was demonstrated that HRS might cure retinal I/R damage, and can meaningfully decrease angiogenesis of cornea after an alkali-burn wound in a rat model [57].
Reported that breathing in of H2 gas at low concentrations (1.3 vol/100 vol) decreases oxidative stress, infrequent hypoxiainduced dyslipidemia, and cardiomyocyte hypertrophy and perivascular fibrosis in left ventricular myocardium of C57BL/6J mice [6,58]. As a curative therapeutic gas, H2 could alleviate various illnesses comprising Parkinson ’s disease, sepsis, acute pancreatitis, and obstructive jaundice by employing an antioxidant action and decreasing ROS [59]. Chen et al. [60] indicated for the first time that high concentrations of hydrogen (HCH) gas (67% H2 and 33% O2), could prevent the myocardium from I/R damage in vitro and in vivo through motivation of the Phosphatidylinositol-3-Kinase (PI3K/Akt) signaling pathway in male C57BL/6 mice. Experimental animal models in dentistry have confirmed that HRW can decrease gingival oxidative stress caused because of periodontal illness and aging [61]. Similarly, a current investigation has shown that ingestion HRW triggered the nuclear factor-E2 related factor 2 (Nrf2)/antioxidant protection route and the expression of the gene of antioxidants, supporting to speeding up of oral mucosal impairment remedial in rats [62]. Furthermore, a study showed that consumption HRW could suppress gingival oxidative stress caused by obesity, and therefore avert alveolar bone resorption in rat models [63].
The outcome of the study has indicated the future of H2 as an active radiotherapy defense, without identified deleterious properties, successfully mitigated the harshness of dermatitis and enhanced skin cells rescue in male Sprague-Dawley rats [64]. Likewise, the results of other work demonstrated that HRW had a therapeutic property on acute radiation-induced skin wounds in rats and that the influences were dependent on the amount of H2 treatment [65,66]. These researchers predicted that the mechanism of action was associated with decreasing oxidative damage, preventing the inflammatory reaction, and stimulating expression of the growth factor [65]. An in vitro scuff assessment exhibited that HRW accomplished cytoprotection from oxidative damage in cultured human gingival fibroblasts or three-dimensional tissue counterparts and injury remedial, ROS declining and diminish glutathione depletion [67]. The preventive properties of H2 on high sugar and high-fat diet (HFD) induced Nonalcoholic fatty liver disease (NAFLD) could be documented to its anti-oxidative effects, in addition to its motivation of peroxisome proliferatoractivated receptor (PPARα and PPARγ) as shown in male Sprague Dawley rats model [67]. Substances containing H2, comprising hydrogen sulfide (H2S) and HRS have been demonstrated to enhance the role of brain salvage after traumatic brain injury (TBI) [68]. Moreover, the ingestion of HRW protects anxiety-induced cognitive deterioration and the production of superoxide in mice [69]. Consumption of HRW inhibited the concentrations of serum ROS and oxidized low-density lipoprotein-cholesterol (ox-LDL), the rate of lipid deposition in the aorta, and the degrees of nitrotyrosine and hexanoyl- lysine (HEL) development in the aorta in rat ligature-induced periodontitis [70].
Numerous substitute pathways are nowadays developed as central representations for energy moderating properties of H2, comprising: (1) ghrelin-linked upregulation of ghrelin receptor (GHS-R1α); (2) ghrelin - linked motivation of glucose transporter 1 (GLUT1); (3) non-ghrelin linked stimulation of glucose transporter 4 (GLUT4); and (4) non-ghrelin linked improved expression of fibroblast growth factor 21 (FGF21), a regulator of energy expenses [71,72]. These mechanisms might be at least partly accountable for the promising influence of H2 on animal and humans illnesses with decreased cellular bioenergetics [73]. A preliminary investigation of HRW treatment in Parkinson’s disease (PD), patients have revealed encouraging results, and it has been stated that HRW displays neuroprotective properties in the murine 1-methyl-4- phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) induced PD mouse model [74,75]. As indicated by a study by consumption of 500 mL of the HRW every day for four weeks, oxidative stress was successfully declined and the infection condition in rheumatoid arthritis (RA) was meaningfully improved [18,76]. HRS (5 mL/kg, daily) suppressed oxidative stress, inflammatory cytokine formation, and nuclear factor kappa-B (NF-κB) manufacture in the hippocampus and cerebral cortex, and enhanced decreased memory in a rat model of Alzheimer’s disease (AD) [77]. The current experimental investigations by Lin and collogues [78] reported that HRW decreased oxidative stress in individuals with metabolic syndrome and chronic hepatitis B.
Presently, the suppression of inflammation by H2 is becoming widespread [79]. The quick spreading, high penetrability, and absence of clear side effects of H2 implies that it is extremely efficient for decreasing ROS radicals, and it has been recognized to be defensive against damage to numerous tissues and organs comprising the heart, liver brain and lung [80,81]. Pointed out that HRW has been broadly observed for its capacity to inhibit reactions of inflammation and mitigate neuronal apoptosis [82]. The study revealed that H2 gas breathing in during ex vivo lung perfusion (EVLP) of the donation after cardiac death (DCD) lungs ameliorates lung role via the activity of anti-inflammation, anti-oxidases, and anti-apoptosis, and those useful properties continue post-transplantation in a pig and rat model [81,83]. Moreover, reported that inhalation H2 can reduce seawater instillation which caused severe lung damage by improving hypoxemia, decreasing inflammatory events, relieving the degree of oxidative damage, and suppressing lung cell apoptosis in rabbits [84].
In a paradigm of hepatic I/R damage, inhalation of H2 protected liver cell death and declined liver malondialdehyde (MDA) and serum alanine aminotransferase (ALT) concentrations [85]. H2 ventilation meaningfully enhanced the survival degree of septic mice in a concentration and timedependent way, in a mouse paradigm of inflammation [86]. Another interesting point is that 2% H2 therapy had useful properties on sepsis and sepsis-associated organ injury, as shown by declined rates of oxidative yields and improved anti-oxidant enzyme action [87]. Likewise, H2 therapy has been described to defend mice from various organ impairment in a zymosaninduced general model, relatively by decreasing the concentrations of proinflammatory cytokine in serum [88]. HRS reduces the concentration of cytokines like interleukin-4 (IL-4), interleukin-5 (IL-5), interleukin-13 (IL-13) and tumor necrosis factor- alpha (TNF-α) in bronchoalveolar lavage fluid. The influences HRS on TNF-α, interleukin- 1 β (IL-1β) and interleukin- 6 (IL-6) amount was assumed as the reason for its preventive effect against ultraviolet B (UVB) radiation [89]. Proven that H2 administration can prevent mice from fatal graft versus host disease (GVHD) and ameliorate clinical disorder of a GVHD [90]. Likewise, H2 might stimulate the regain of white blood cells (WBCs) of GVHD mice [91]. H2 may be also possibly an efficient remedy for GVHD by downregulating cytokines and selectively neutralizing •OH and ONOO- radicals [92], since these cytokines comprising IL-6, IL-1β, TNF- α and free radicals have been anticipated to play significant parts in the development and progression of GVHD.
A number of investigations have provided evidence from animal studies that indicated HRS can mitigate intestinal infections such as intestinal I/R damage, ulcerative colitis (UC), and colon inflammation [93]. The outcomes of the research demonstrated that HRW can reduce the signs and colonic injury in dextran sulfate sodium (DSS) -induced inflammatory bowel disease (IBD), most probably because of its exceptional cytoprotective effects perhaps its anti-inflammatory and anti-oxidant actions [94]. Interestingly, HRW can prevent ER stress and up-regulated heme oxygenase-1(HO-1) expression. Therefore, their findings pointed out that HRW could be a possible treatment for DSScaused IBD [94]. Zhang and coworkers have stated that pretreatment with HRW might alleviate aspirin-induced stomach injuries by suppression of the inflammatory responses in mice model [95]. The study provided confirmation that the administration of HRW could prevent Wistar rats from chlorpyrifos (CPF)-induced toxicity of neurons, and the preventive properties of H2 might be facilitated by its antioxidative action [96]. Reported the prospective antiinflammatory properties of H2 gas in prolonged liver inflammation caused by the parasite Schistosoma mansoni in mice model [97]. Administration of H2 to hemodialysis (HD) solutions seems to recover inflammatory reactions and improve blood pressure (BP), providing a unique treatment opportunity for uremia regulation in HD patients [98]. In another investigation, intense physical exercise-brought decrease in muscle function in athletes was enhanced by drinking HRW [99]. Zhang and colleagues [100] verified that inhalation of H2 gas could reduce air route inflammation by ameliorating lung role in an asthmatic BALB/c mouse model.
A contemporary study has displayed that HRS can successfully prevent Endoplasmic reticulum stress (ERS) [101]. Their results suggested that HRS administration can prevent liver ischemiareperfusion injury (IRI) by decreasing extreme ERS, thus mitigating ERS-induced apoptosis. A study explored the possible way of the preventive influence of HRS on doxorubicin and exhibited that HRS therapeutic might hinder the inflammatory cytokines like tumor necrosis factor-alpha (TNF-) and interleukin -6 (IL-6) pathway, elevate the fragmented C8 expression and the ratio of Bcl-2/Bax, and weaken cell apoptosis in liver and heart tissue [79]. In a study, the influence of H2- silica on cell migration was examined, and the detail obtained was that H2-silica is more effective in preventing the movement of malignant cells than normal cells [102,103]. They proposed that H2-silica could hinder the metastasis of cancer cells in vitro. The useful influence of drinking HRW in the inhibition of diabetes and resistance of insulin has been reported in a human study [104]. A study has shown that H2 reacts with •OH, and therefore might protect damage triggered by ROS, by the mechanisms of inhibition of mitogen-activated protein kinase (MAPK), nuclear factor-kappa B ( NFkB), and caspase-3, and consequent apoptosis suppression [104]. Another study revealed an enhancement of glucose and lipid oxidation when HRW was given to patients with type 2 diabetes or compromised glucose tolerance [105,106]. An investigation confirmed that H2 repressed radiation-induced caspase-3 and B-cell lymphoma wounds associated protein X (Bax) stimulation, and improved Bcell lymphoma 2 (Bcl-2) concentrations after cerebral I/R in rat models [107]. H2 appears to trigger the PI3K /AKt pathway and decrease neuronal apoptosis through the control of the Bcl-2 family and caspase -3 in a model of acute brain damage after hemorrhage of subarachnoid [108]. Current studies by Gao and colleagues reported that HRW had antidepressant similar properties in chronic mild stress (CMS) administered mice by suppressing apoptosis, oxidative stress and inflammation in the hippocampus and prefrontal cortex [109]. It was noted that inhalation of 2% H2 significantly mitigates liver damage and apoptosis by decreasing lipid peroxidation and the level of MDA [110].
Novel Advantages of H2
To date, inadequate information exists regarding pharmacodynamics and toxicity of H2 [11]. At present, the therapeutic effect of H2 is already recognized in a medical field, nonetheless, numerous perplexities are until to be fixed before its acknowledgment as a harmless and effective remedial gas [11]. H2 has numerous potential benefits as a valuable treatment agent in clinical medication. The physical capacity and small molecular mass allow its quick dispersal through the plasma membrane to cytosol and other target cells and sub-cellular compartments [28,103]. The physiological variables such as oxygen saturation, temperature, pH, and blood pressure are not influenced by the delivery of H2 [11,67,111].
The outcome of H2 in biomedical science seems to be like other kinds of therapeutic gas families like NO, hydrogen sulfide (H2S), and carbon monoxide (CO) which were once regarded as not essential [11]. H2 was regarded as unreactive gas just a decade before; nowadays the scientific community realizes H2 as a healing agent with very widespread use preferences in medication [112]. Even though the existing published pieces of information on H2 are still inadequate, the promising properties of H2 treatment established in the mainstream of pilot trials motivate upcoming study; appreciating the activities of H2 could guide us to novel types of H2 remedy for many humankind disease scenarios.
The use of H2 is reasonably hopeful for the therapy of several chronic and acute disease situations, along with its value in the provision of good health conditions. The non-toxic and quick sub-cellular diffusion features of this medical agent guarantee the practicality and propensity for its clinical application. Therapeutic or protective use of H2 has been done by diverse administration approaches comprising inhalation of H2 gas, ingestion of HRW or injection of HRS. It is very imperative to elucidate the best delivery method and ideal H2 dosage for every disease model preclinically and consequently in the patient population. H2 reacts with strong oxidants, like •OH and ONOO- radicals that make it promising to use its potential for protective and healing practices. H2 is capable of acting as an antioxidant, anti-inflammatory, and anti-apoptotic agent. This review summarizes presently existing information concerning the preventive and therapeutic roles of H2 in various animal models and human pathologies associated with oxidative stress, inflammation, and apoptosis. Further studies are needed with expanding the basic concepts and researches of H2 into clinical uses.
The authors have stated that no conflict of interest occurs.
Citation: Abisso TG, Adzavon YM, Zhao P, Zhang X, Liu M, Ma X, et al. (2020) Current Progress In Molecular Hydrogen Medication: Protective And Therapeutic Uses Of Hydrogen Against Different Disease Scenarios. Intern Med 10:314. doi: 10.35248/2165-8048.20.10.314
Received: 27-Dec-2019 Accepted: 13-Jan-2020 Published: 20-Jan-2020 , DOI: 10.35248/2165-8048.20.10.314
Copyright: © 2020 Abisso TG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.