Journal of Bone Research
Open Access
ISSN: 2572-4916
+44 1478 350008
ISSN: 2572-4916
+44 1478 350008
Short Communication - (2022)Volume 10, Issue 1
Well-established regimens of standard chemotherapy are considered the standard of care for patients with advanced or metastatic osteosarcoma, but results are generally disappointing. In the past few years, however, novel treatments have been tested in clinical trials and deserve to be comparatively evaluated. Our objective was to review the current data of effectiveness for these new agents based on the end-point of Overall Survival (OS). The Shiny technique was employed for reconstructing individual patient data. A standard Cox statistics was run to estimate the Hazard Ratios (HRs) for pairwise comparisons.
After a standard literature search, four new treatments were identified (regorafenib, cabozantinib, apatinib plus camrelizumab, pembrolizumab). Evidence on effectiveness for these treatments was available from 5 phase-II clinical trials; Kaplan-Meier curves of OS were available for all treatments except pembrolizumab. Total numbers of evaluable patients were 26, 22, 42, and 43 for regorafenib, cabozantinib, apatinib plus camrelizumab, pembrolizumab, respectively. Pembrolizumab could not be evaluated owing to the lack of OS curve. Gemcitabine plus sirolimus (for a total of 35 patients) was considered the control treatment in terms of standard chemotherapy.
Using gemcitabine plus sirolimus as common comparator, each of the three novel treatments (regorafenib, cabozantinib, apatinib plus camrelizumab) showed no significant improvement in OS. The only statistical trend (at p=0.06) was found for regorafenib that showed a numerical improvement in OS compared with controls.
Our analysis indicates that these novel treatments for osteosarcoma, which share a similar efficacy with one another, provide no significant improvement in OS compared with standard chemotherapy. Further research is needed to identify other agents determining a more substantial OS improvement.
Osteosarcoma; Pemetrexed; Regorafenib; Cabozantinib; Apatinib plus camrelizumab; Gemcitabine plus sirolimus; Reconstruction of patient-level data
In advanced or metastatic osteosarcoma, chemotherapy based on cytotoxic agents is considered the standard of care, but novel treatments have been proposed in recent years [1-5]. Our objective was to synthesize the state of the art about these novel treatments by analyzing their effectiveness and by comparing them with one another. These analyses were designed as indirect comparisons. Survival data were handled by using an original technique (the Shiny method) that reconstructs individual patient data from Kaplan-Meier curves [6]. The Shiny method, which is the evolution of a similar method developed in 2011 by Guyot, et al. [7], has the advantage of being freely available on the Internet.
Overall Survival (OS) was the end-point of our analysis. A standard literature search was conducted on PubMed and EMBASE to identify clinical trials (either phase II or phase III) that tested the effectiveness of new agents when given to patients with advanced or metastatic disease as second or further line. The availability of a Kaplan-Meier curve of OS was an inclusion criterion. Our analyses took into account that techniques of reconstruction of individual patient data from Kaplan-Meier curves are highly reliable [6,7]. Among these, the Shiny method has demonstrated the best operational performance [6]. Its procedure generates a patient database in which each individual is assigned the length of follow- up and outcome (expressed as either censored or with event). This reconstruction is carried out based on the Kaplan-Meier graph along with the total number of enrolled patients and deaths. In the analysis of included trials, after reconstructing patient data, we carried out standard Cox statistics based on time-to-event endpoint (event=death for any cause). Pairwise indirect comparisons were handled based on the Hazard Ratio (HR) with 95% Confidence Interval (CI). Calculations were carried out under the R-platform; statistical significance was set at p<0.05.
Five trials [1-5] were identified through our literature search (Table 1). All were phase II trials. Four treatments were tested in these trials (regorafenib-2 trials, cabozantinib, apatinib plus camrelizumab, and gemcitabine plus sirolimus). To reconstruct individual patient data, the Shiny technique was applied to the 6 Kaplan-Meier curves (Table 1). As a control group for our indirect comparisons, we considered gemcitabine plus sirolimus. Two trials investigated regorafenib; since these two Kaplan-Meier showed a nearly identical pattern (data not shown), individual patient data from these two trials were pooled together to generate a single cohort.
| First author and year of publication | Inclusion criteria | Treatment | No. of patients | No. of deaths |
|---|---|---|---|---|
| Duffaud, et al. [1], 2019 | Patients aged 10 years or older with histologically confirmed osteosarcoma whose disease had progressed after treatment with one to two previous lines of chemotherapy for metastatic disease and an Eastern Cooperative Oncology Group performance status of 0 or 1. | Regorafenib | 26 | 21 |
| Davis, et al. [2], 2019 | Patients with progressive metastatic osteosarcoma with measurable disease by RECIST who had received at least one prior line of therapy. | Regorafenib | 22 | 12 |
| Italiano, et al. [3], 2020 | Patients aged 12 years or older, Eastern Cooperative Oncology Group performance status of 0-1, and documented disease progression (according to Response Evaluation Criteria in Solid Tumors version 1.1) before study entry. No limits in the number of previous lines of treatment#. | Cabozantinib | 42 | 32 |
| Xie, et al. [4], 2020 | Patients with histologically confirmed metastatic or locally advanced osteosarcoma, as reviewed by the Pathology Committee of Peking University People’s Hospital and not eligible for curative-intent surgery. No response to previous systemic chemotherapy, including HD-MTX, ADM, and DDP with/without IFO. | Apatinib plus camrelizumab | 43 | 28 |
| Martin-Broto, et al. [5], 2017 | Metastatic osteosarcoma patients, relapsed or progressing after standard chemotherapy and unsuitable for metastasectomy. | Gemcitabine plus sirolimus | 35 | 22 |
Note: Monotherapy with pembrolizumab has been evaluated in a trial conducted by Boye, et al. (12 patients, 11 deaths), in which the Kaplan-
Meier curve of OS has not been reported (ClinicalTrials.gov, number
NCT03013127).
#Although the presence of metastasis was not reported as an explicit inclusion criterion, more than 90% of included patients had metastatic
disease.
Table 1: Advanced or metastatic osteosarcoma in pretreated patients: Information on treatments reported in 5 phase II trials.
Figure 1 shows the Kaplan-Meier graph of for the 4 treatments. Using gemcitabine plus sirolimus as common comparator, each of regorafenib, cabozantinib, and apatinib plus camrelizumab showed a numerical improvement in OS that however did not reach statistical significance. To better quantify OS benefit we carried out one further analysis on a time scale in which we compared regorafenib, cabozantinib, and apatinib plus camrelizumab pooled together vs. gemcitabine plus sirolimus; median OS was 11.2 vs. 7.4 months (gain=3.8 months); HR was 0.63 (95% CI, 0.40 to 0.97, p=0.04).
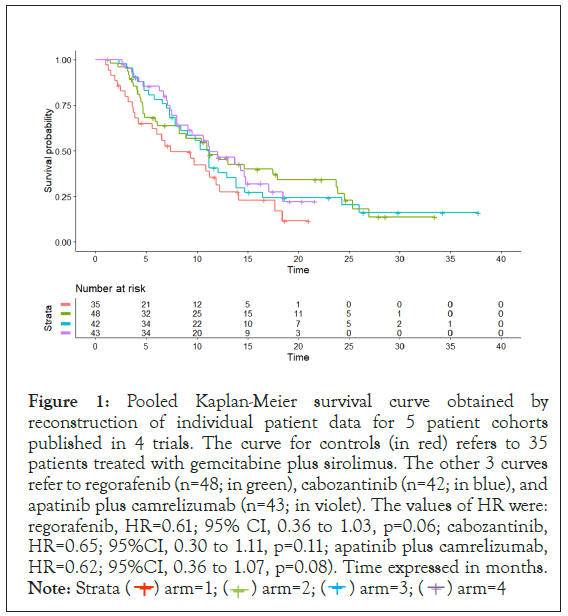
Figure 1: Pooled Kaplan-Meier survival curve obtained by
reconstruction of individual patient data for 5 patient cohorts
published in 4 trials. The curve for controls (in red) refers to 35
patients treated with gemcitabine plus sirolimus. The other 3 curves
refer to regorafenib (n=48; in green), cabozantinib (n=42; in blue), and
apatinib plus camrelizumab (n=43; in violet). The values of HR were:
regorafenib, HR=0.61; 95% CI, 0.36 to 1.03, p=0.06; cabozantinib,
HR=0.65; 95%CI, 0.30 to 1.11, p=0.11; apatinib plus camrelizumab,
HR=0.62; 95%CI, 0.36 to 1.07, p=0.08). Time expressed in months.
The main finding arising from our analysis is that the novel treatments proposed as second or further line for advanced or metastatic osteosarcoma have similar efficacy and, more importantly, do not provide any substantial survival benefit compared with the standard of care (gemcitabine plus sirolimus). One strength of our analysis is the excellent performance of the Shiny method [6]. Another one is represented by the original design of indirect comparisons based on reconstructed individual data. This strategy of evidence analysis permits to indirectly compare new agents with one another in cases where direct comparisons based on “real” trials are not available.
As regards weaknesses of our analysis, the small number of subjects enrolled in included trials should be pointed out. Another one is that these indirect retrospective comparisons do not take into account the effect of unavoidable intrinsic differences in the patient cohorts.
In conclusion, as regards novel treatments for advanced or metastatic osteosarcoma, our paper has presented the current state of the art in which no treatment is characterized by better efficacy compared with the others. Overall, these new treatments unfortunately do not determine any substantial clinical benefit in terms of OS. Further research should therefore be conducted in this field because the achievement of better outcomes in metastatic osteosarcoma continues to be an unmet need.
The author declares no conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Messori A (2022) Current Treatments for Advanced or Metastatic Osteosarcoma: Indirect Comparisons Based on Individual Patient Data Reconstructed Retrospectively from 5 Trials. J Bone Res. 10:156.
Received: 03-Jan-2022, Manuscript No. BMRJ-22-15537; Editor assigned: 05-Jan-2022, Pre QC No. BMRJ-22-15537 (PQ); Reviewed: 18-Jan-2022, QC No. BMRJ-22-15537; Revised: 24-Jan-2022, Manuscript No. BMRJ-22-15537 (R); Published: 31-Jan-2022 , DOI: 10.35248/2572-4916-22.10.157
Copyright: © 2022 Messori A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.