Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Short Communication - (2024)Volume 15, Issue 6
Cutaneous Leiomyoma (CL) is benign tumour arises from smooth muscle cells. Clinical presentation can range from solitary, multiple, segmental and zosteriform. These painful tumours tend to occur more frequently in adults. They can be either inherited or sporadic. Multiple CL can be associated with reed syndrome. This condition has a significant association with internal organ involvement, such as the uterus, kidneys, and retroperitoneal soft tissues. This condition can mimic many other disorders; therefore, histopathological examination combined with special stains is crucial for an accurate diagnosis. Medical treatment is often unsatisfactory; however, surgical options are generally preferred for managing solitary lesions and angioleiomyomas. Multiple cutaneous leiomyomas can substantially affect patients' quality of life and result in significant cosmetic concerns. Although sarcomatous transformation is rare in cutaneous leiomyomas, clinicians should remain vigilant for both sarcomatous changes and systemic associations. Hence detailed clinical evaluation and screening should be undertaken for early detection of complication.
Cutaneous leiomyomas; Smooth muscles; Soft tissue tumour; Reed syndrome; Pilar leiomyoma; Dermatopathology; Appendageal tumour
Leiomyomas are rare form of benign smooth muscle tumours. The most common site of occurrence is the uterus (95%), followed by the skin (3%) [1]. Cutaneous Leiomyoma (CL) is a rare benign tumour that originates from the smooth muscle cells. They comprise of 5% of all leiomyoma and accounts to 75% of all extrauterine leiomyoma [2]. These are the slow growing tumours. They can occur in 9-60 years of age [3-5]. These tumors are most commonly seen in adults, typically between the fifth and sixth decades of life, whereas Piloleiomyomas (PLM) are most frequently found in individuals aged 10 to 30 years.
CL can be classified based on number of lesions as solitary and multiple [6,7]. The multiple CL can be generalized, segmental or zosteriform (Figure 1). Based on site of origin classified as arrector pili, muscle of the hair follicle (Piloleiomyoma/PLM), tunica dartos of the scrotum, mamillary muscle of the nipple (Genital leiomyoma/GLM), and the smooth muscle of the blood vessels (Angioleiomyoma/ALM). Based on presentation they can be of zosteriform (segmental) distribution, neurofibroma like presentation. The segmental distribution can be subdivided as type 1, type 2 by happle [8]. Type I segmental leiomyomas result from heterozygous post-zygotic mutations, leading to segmental skin lesions that are similar to those seen in a non-mosaic phenotype. Type II segmental leiomyomatosis results from a post-zygotic mutational event in a heterozygous embryo, leading to loss of heterozygosity. This process produces a distinct pattern of segmental lesions that overlays the typical phenotype of the underlying disease [9]. Type 1 segmental leiomyoma typically presents as a single unilateral segmental involvement, while type 2 involves multiple unilateral segments and may include nonsegmental lesions on the contralateral side.
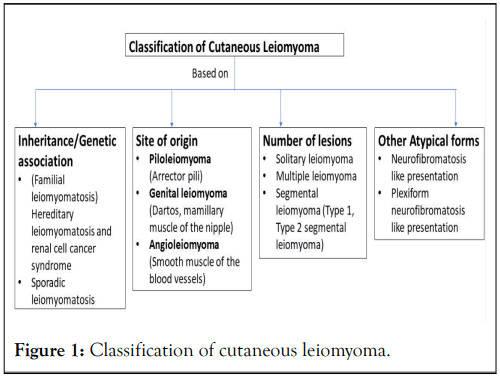
Figure 1: Classification of cutaneous leiomyoma.
Clinically CL presents single or multiple painful firm tender skin to brown coloured papules, nodules or swelling (Figures 2 and 3). Sometimes diffuse involvement of entire skin in the form of indurated plaque of particular anatomical site. The size of CL can be varied from 10 cm-30 cm while trunk being the most common site [10,11]. Pseudo Darier sign, a transient piloerection or elevation of the nodule induced by rubbing or contact with ice cube may be present. The multiple lesions can be distributed either segmental or generalised distribution [7,12]. The few reports of CL were clinically resembled the neurofibromatosis [11,13].
Figure 2: (a and b) There were multiple pink to reddish brown tender globular swellings and nodules of variable sizes present over trunk (a and b) with diffuse involvement of back in the form of indurated plaque (b).
Figure 3: (a,b) Multiple papules and shiny skin to browncoloured nodules of size varied from 5 mm × 10 mm to 20 mm × 25 mm predominantly involving multiple left thoracic and lumbar segments. The lesions at lumbar segments were coalescing to form sheet like plaque. Few of nodules were scattered at right side of the back (b).
Genital Leiomyomas (GLM), typically develop on the genitals, including the scrotum, penis, and vulva. However, they may also be found on the nipple-areolar complex. The lesions are generally an asymptomatic, solitary nodule, usually smaller than PLM. Anecdotal report had giant plaque of size of 30 cm at labia major [10]. Occasionally, genital leiomyomas may present as pedunculated lesions, which can mimic acrochordons or genital warts.
Angioleiomyoma (ALM) accounts for one-fourth to one-half of all superficial leiomyomas. It usually found in the subcutical layer and is composed of many thick walled vessels, unlike cutaneous leiomyoma, which has few thin-walled vessels in it. It is well-circumscribed than cutaneous leiomyoma and commonly occurred between age from 40 to 60 [14]. These CL commonly present as solitary painful nodule/swelling at lower extremities. The pain is aggravated with pressure or temperature change. Histologically, vascular leiomyoma can be classified into three groups, capillary or solid (67%), venous (23%) and cavernous (11%) type [15]. The pain in CL could be because of direct impingement of the tumour on the cutaneous nerves. Other causes could be the infiltration of mast cells, muscle contraction, local vasoconstriction leading to muscle contraction [16]. This is often aggravated by touch, cold, emotional stress. The character of pain is typically paroxysmal with burning, pinching or stabbing quality.
Multiple Piloleiomyomatosis (MPL) can be associated with various conditions, including Reed's syndrome, polycythemia, and visceral involvement, particularly affecting the gastrointestinal tract and retroperitoneal area. A few cases of MPL have also been associated with multiple endocrine neoplasia type 1, rheumatoid arthritis, as well as breast, prostate, and bladder cancers, in addition to adrenal cortical adenomas and renal and ovarian cysts. The most concerning association among them is Reed's syndrome, also known as Hereditary Leiomyomatosis and Renal Cell Cancer syndrome (HLRCC) [11,17-19]. This arises from a mutation in the gene that encodes fumarate hydratase, located on chromosome 1q42.3.43. The most common manifestation of HLRCC include multiple cutaneous and uterine leiomyomas in 76%-100% of cases [20]. The RCC was observed in 10%-16% type 2 papillary renal cell carcinoma is most common tumour associated with HLRCC. When piloleiomyomas arise due to a genetic syndrome, they typically present earlier in life, with an average age of onset around 25 years, though this can range from 10 to 50 years [21]. The precise mechanism by which heterozygous fumarate hydratase deficiency leads to leiomyoma development is not fully understood. However, it is suspected that fumarate hydratase may serve, at least partially, as a tumor suppressor. According to Alam, et al., approximately 89% of patients with multiple cutaneous leiomyomas have a heterozygous germline mutation in the Fumarate Hydratase (FH) gene [22].
Both major and minor clinical diagnostic criteria have been established to assist in the "likely" diagnosis of hereditary leiomyomas and renal cell carcinoma.
Major criteria
The presence of multiple histologically confirmed piloleiomyomas.
Minor criteria
• The surgical treatment for uterine leiomyomas before age 40.
• Type 2 papillary renal cell carcinoma before age 40.
• First-degree family member who meets one of the previously mentioned criteria.
The diagnosis of HLRCC should be strongly considered if a patient displays either the 1 major diagnostic criteria or 2 of the 3 minor criteria [21].
The heterozygous FH is not considered as either a major or minor criteria because on rare occasions patients affected with HLRCC may test negative for a fumarate hydratase deficiency. In such scenarios, either the piloleiomyomas or the uterine leiomyomas may be immunohistochemically stained to evaluate for excessive accumulation of fumarate. A fumarate enzyme assay may also be performed to evaluate for fumarate hydratase deficiency, and FH activity less than 60% of normal is indicative of HLRCC.
The diagnosis of CL (Cutaneous Leiomyoma) can be confirmed clinically and is often supported by dermatoscopy. Dermatoscopic examination typically reveals discrete brown reticular lines along with numerous whitish clods of varying size and number. The few lesions may reveal tortuous/dilated capillaries (Figure 4) [11]. However, the histopathological examination is confirmatory. This is characterized a circumscribed, non-encapsulated tumour with a grenz zone beneath the epidermis (Figure 5). The dermis comprised of interlacing fascicles with wavy muscle fibres. The individual spindle cells exhibit eosinophilic cytoplasm and elongated nuclei, with a subset may demonstrate a perinuclear halo without cellular atypia.
Figure 4: (a,b) Dermatoscopy revealed discrete brown reticular lines, along with numerous whitish clods of variable size and number, with few lesions (b) may show dilated/tortuous capillaries.
Genital leiomyoma has more circumscribed and more cellular as compared to PLM [14]. Angioleiomyomas (ALM) usually found in the subcutical layer. That demonstrate smooth muscle cells with slit-like or dilated vascular channels. Histologically, these lesions are classified into three groups: Capillary or solid (67%), venous (23%), and cavernous ALM (11%) [15].
Masson's trichrome stain, Smooth Muscle Myosin (SMM), Van Gieson stain, and immunohistochemical stains for smooth muscle markers such as actin and desmin (Figure 5) may be necessary for accurate histological assessment. Additional immunohistochemical testing may be conducted to detect fumarate hydratase deficiency in the specimen or to identify the accumulation of precursor substances. Masson's trichrome stain revealed that the smooth muscle cell cytoplasm stained red, whereas the collagenous fibrous tissue stained blue. These presentations are difficult for clinical diagnose. Other potential differential diagnoses to consider are eccrine spiradenoma, neuroma, dermatofibroma, angiolipoma, neurilemmoma, endometrioma, glomus tumour, granular cell tumour, lipoma, cylindroma, eccrine poroma, and angiofibromas related to tuberous sclerosis.
Figure 5: (a): Histopathological examination (Haematoxylin and Eosin stain, 100x) revealed minimal epidermal atrophy with increased in basal layer pigmentation. The papillary dermis (b) (400x) showed band of relative pale zone with few dilated capillaries, which was suggestive of Grenz zone. Both upper and deeper dermis showed (b and c) (400x) circumscribed, nonencapsulated tumour with interlacing fascicles with wavy muscle fibres. Individual spindle cells show (c) eosinophilic cytoplasm with elongated nuclei and blunt ends. There were no cytological atypia or mitosis. These features were consistent with benign cutaneous piloleiomyoma. (d and e) On immunohistochemistry (400x), cells were positive for smooth muscle actin. (f) Spindle cells and epidermis stained pink whereas adjacent collagen stained blue on Mason’s trichrome stain (100x).
Effective treatment of symptomatic piloleiomyomas is often unsatisfactory for both physicians and patients because of the high recurrence rate following surgical intervention and the limited success of pharmacological treatment options [21]. The treatment approach depends on the extent of involvement, the size of individual lesions, and the severity of symptoms. The condition is often resistant to medical treatment. The pain alleviation is challenging task for clinician. Camouflage with cosmetics and avoidance of exposure to cold may be the only measures required. Surgical excision with skin grafting may be indicated for a small group of lesions. Destructive methods like radiofrequency ablation, carbon dioxide laser ablation, cryotherapy and electrodessication had been tried [4,23]. The condition may however recur, particularly in patients with multiple lesions. When lesions are painful, various pharmacologic agents such as nifedipine, phenoxybenzamine, gabapentin, pregabalin, duloxetine, doxazosin, alpha-1 blockers and nitroglycerin, topical lidocaine or caispaicin or intralesional corticosteroid or botulinum toxin may be tried alone or in combination [4].
Patient’s as young as ten years old have been diagnosed with HLRCC associated renal cell carcinoma, which makes screening at a young age essential. Children should be initially screened for fumarate hydratase deficiency between the ages of 8 to 10, and if positive, they should receive an annual MRI to screen and monitor for the development of renal cell carcinoma. The same protocol stands true for adults diagnosed with HLRCC with positive fumarate hydratase mutation. In addition, patients should undergo a complete physical and gynaecological exam (if indicated) yearly, along with dermatological examinations biannually. First-degree family members of affected patients should also be appropriately screened for HLRCC with genetic testing for fumarate hydratase deficiency, along with appropriate abdominal or pelvic screening [11].
Both radiological and clinical screening is needed in patient with multiple CL. MRI (Magnetic Resonance Imaging) of renal system should be done either annually or once every 2 yearly for early detection of RCC. Consequently, it's recommended to perform annual screenings for both patients and their family members. Clinical screening should focus on possible sarcomatous change in existing lesions. Even though CL rarely undergo sarcomatous change. As cutaneous leiomyosarcoma constitutes about 2-3% of all cutaneous soft tissue sarcoma [24].
In conclusion cutaneous leiomyoma is uncommon entity with varied clinical presentation. The clinician should be aware of such uncommon tumour. Dermatoscopic and histopathological examinations play a crucial role in confirming the diagnosis. A holistic screening of patients and family members is needed for early intervention to prevent mortality.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Antakanavar GM, Kumar LP, Yadav A, Hasan Z, Barman KD, Sahoo B, et al. (2024) Cutaneous Leiomyoma with Varied Presentations: An Update and Review. J Clin Exp Dermatol Res. 15:673.
Received: 28-Oct-2024, Manuscript No. JCEDR-24-33721; Editor assigned: 30-Oct-2024, Pre QC No. JCEDR-24-33721 (PQ); Reviewed: 13-Nov-2024, QC No. JCEDR-24-33721; Revised: 22-Nov-2024, Manuscript No. JCEDR-24-33721 (R); Published: 29-Nov-2024 , DOI: 10.35841/2155-9554.24.15.681
Copyright: © 2024 Antakanavar GM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.