Journal of Cancer Science and Research
Open Access
ISSN: 2576-1447
ISSN: 2576-1447
Research Article - (2022)Volume 7, Issue 5
The rising incidence of primary cutaneous melanoma and higher mortality rates associated with melanoma makes this issue a significant concern globally. A cure is possible with early detection of the disease. Biopsy techniques used for diagnosing a clinically suggestive lesion of melanoma are described, as per recommendations of the American Joint committee on Cancer (AJCC) system. CMs are extracted, keeping safe margins of 1-2 cm. Advancement in the laboratory, molecular, and imaging techniques is inspected in the melanoma cases which are newly diagnosed. For treatment strategies of primary cutaneous melanoma, recommendations for surgical margins and the excision techniques are discussed. The importance of sentinel lymph node biopsy as a staging technique for cutaneous melanoma is discussed in detail, with recommendations for its clinical practice efficiency. For cases of distant metastasis, all alternatives to surgical therapy should be considered together. However, systemic treatment is indicated in the absence of surgical therapies. First-line treatment in wild type B-RAF proto-oncogene (BRAF) patients, immunotherapy with programmed death-1 (PD-1) antibodies alone or combination therapy of PD-1 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) antibodies should be preferred. Inhibitors of BRAF, such as dabrafenib and vemurafenib, combined with the mitogen-activated protein kinase (MEK) inhibitors trametinib and cobimetinib for BRAF mutated patients, should be considered for treatment. Finally, data regarding melanoma, testing, and management related to novel targeted agents and immunotherapies for advanced disease cases are summarized.
The most perilous type of skin cancer, i.e., Cutaneous Melanoma (CM), develops in the skin’s epidermal cells, which are termed as melanocytes. Melanocytes are neural crest-derived cells found in the basal epidermis and hair follicles, along mucosal surfaces, meninges, and in the choroidal layer of the eye [1]. In response to UV, skin keratinocytes produce the melanocyte-stimulating hormone, which binds the melanocortin receptor one on the melanocytes that produce and release melanin. The melanin pigment acts as a shield for UV radiation, thereby preventing DNA alteration [1]. The most known environmental risk factor for developing CM is ultraviolet radiation (UV) from different sources such as sun and tanning beds. Individuals with lighter skin and hair tone have low melanin levels and are at increased risk of developing melanoma. Additionally, the sunburns accumulated since adolescence in individuals are also at high risk. Moreover, the quantity of moles on an individual’s body expands the risk of CM [2]. Keeping in mind these facts; the world’s highest incidences are in Australia and New Zealand as they are close to the equator, have diminished ozone layers, and higher populations of fair-skin toned people. A positive family history of CM is also at an increased risk due to inherited genetic mutations and sun exposure habits. CDKN2A gene on chromosome 9 in a mutated form in individuals is believed to be at high risk for developing melanoma. Studies claim that 70% of CM cells have affected the CDKN2A gene due to somatic mutations. Under normal circumstances, this gene’s product plays a vital role in suppressing cancer, thereby controlling the growth of tumor cells; however, if this gene gets mutated, the tumor suppressor activity is lost and cancer cells might grow in an uncontrolled manner 2. The current review discusses the advancement in diagnosis, staging, and specific biomarkers associated with melanoma and management strategies.
Epidemiology
CM causes mortality in more than 90% of skin diseases. The overall frequency of Cutaneous Melanoma (CM) has been expanding yearly at a quicker rate than other cancer types. Rising incidence ranks it 15th among most common cancers worldwide. The incidence rate of CM differs significantly among countries and this variation in incidences is attributed to variations in skin phenotype and differences in sunlight exposure 2.
Subtypes of CM
Subtypes of melanoma include superficial spreading, lentigomaligna, nodular, acrallentiginous, desmoplastic, and amelanotic [3]. The superficial spreading subtype is most commonly found in approximately 70% of melanomas. The lentigomaligna subtype is less commonly diagnosed; it is slowly progressing and appears in sun-exposed areas (face, head). Nodular melanomas are identified by the absence of a radial growth phase, robust vertical invasion, and variable presentation. Acrallentiginous melanomas are frequently associated with darker skin tone and commonly found on the palms, soles, and subungual spaces. Desmoplastic melanomas are relatively uncommon and are typically observed in elderly patients. Amelanotic melanomas, the most challenging subtype in diagnosis, have no pigmentation and are rarely diagnosed [4].
Stages of CM
Clinical diagnosis and categorization of staging are made based on the American Joint committee on Cancer (AJCC) system. Staging is determined via analysis of the patient’s tumor, distant metastasis, and the number of metastatic nodes [5]. Terms used for describing staging are early, locoregional, and metastatic. Early stages, i.e., stages 0-II, are the ones that are originated at a primary site. Locoregional refers to the region where the tumor spreads to local lymph nodes (LN) or nearby skin/lymph vessels. Tumor, with this spread, is referred to as stage III. The term ‘’Metastasis’’ refers to the tumor that spreads to other organs and different parts of the body. In such cases, the disease would be labelled as stage IV. Patients diagnosed through biopsy with shallow lesions (4.0 mm) are associated with high risk for the metastatic stage. If metastasis occurs, patients are given a diagnosis of stage III or IV. The most likely non-contiguous regions where CM spreads are the LNs. Sentinel lymph nodes (SLNs) are especially significant as they are the first nodes encountered from the region where the primary CM is situated [2,4]. The presence or absence of CM cells at the SLN is a powerful predictor for recurrence and survival in patients with CM. The skin and subcutaneous tissues are the common areas for metastasis of CM. The first most common sites of visceral metastasis in CM are lungs and pleura, with 10% of cases developing pulmonary metastasis during the disease. The brain is another such site for the metastatic spread in cases with CM. Brain metastasis more likely to develop primary lesions in the head, neck, trunk, or abdomen in CM cases [2,4]. Hepatic metastases are found in 10%–20% of cases with metastatic CM. Skeletal metastasis is uncommonly diagnosed compared to other sites but is still diagnosed in 11%–17% of CM cases. Like skeletal metastasis, the gastrointestinal spread is diagnosed in later-stage disease, with the small intestine being the frequent site. Women are most often diagnosed with CM on an extremity, which is the prime reason for improved overall prognosis compared to males whose CM are located on the head, neck, or trunk. Increased age is associated with a more unsatisfactory outcome. The degree of LN involvement is a potent prognostic factor, i.e., a favourable prognosis is inversely proportional to an increased number of nodes and metastasis. Hepatic, pulmonary, and brain metastasis are often associated with a higher mortality rate than spread confined to different locations. Defining the precise location of metastasis is essential, as it defines treatment options [2].
Current trends in diagnosis:
Skin biopsy remains the initial step to set up an authoritative finding of CM after suspicious findings on dermoscopy, though different molecular and imaging techniques are also known. For a Lesion that is clinically indicative of CM, a complete excision biopsy should be performed, including the entire lesion with negative margins, keeping in mind that the lesion is not histologically cut across the profound margin [6-8]. This can be accomplished using a restricted fringe edge of 1 to 3 mm around the concerning skin lesion [6]. A partial biopsy may incorrectly stage CM at the beginning and could influence treatment planning [6,9-11]. Figure 1 demonstrates the road map of skin cancer diagnosis over the years.
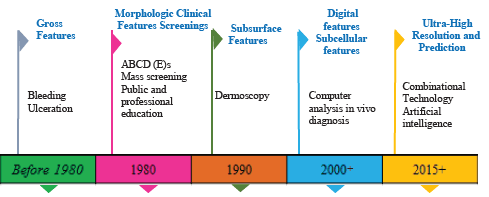
Figure 1: Road map of skin cancer diagnosis over the years
A sentinel lymph node (SLN) biopsy is routinely performed in cases having tumours more than 1 mm thickness. Excisional biopsy in various forms, such as elliptical, punch, and saucerization is performed, amongst which saucerization being the most common as it is more convenient and timesaving. Saucerization should not be confused with a superficial shave biopsy, which is used only during a suspicion of invasive melanoma. Superficial shave biopsies might misjudge Breslow’s thickness, ultimately mislabelling CM’s stage and are thus not encouraged for diagnosis of CM [11,12]. Complete excisional biopsy is difficult to perform in challenging areas such as the face/acral surfaces. Under such circumstances, punch, shave, or elliptical/fusiform incisional biopsy should be performed [13]. It is still not proven that partial/incisional biopsies affect patient outcomes adversely due to the transfer of melanoma cells into blood vessels or cutaneous lymphatics. Incisional vs excisional biopsy types rarely affect SLN or disease recurrence rates, nor does it result in metastasis [10,14]. A biopsy is performed for a suspicious nail lesion (e.g., diffuse pigmentation, melanonychiastriata, or amelanotic changes) after sampling the nail matrix. As nail anatomy is complex and melanoma occurs in the nail matrix, suspicious nail lesions are best assessed and sampled by skilled practitioners. Prebiopsy photos are of significant help to clinical/pathologic connection and help forestall medical procedures at an incorrect site if further therapy is required. Recently, non-invasive techniques such as reflectance confocal microscopy, electrical impedance spectroscopy, gene expression analysis, and optical coherence tomography benefit more due to availability [15-17]. Non-invasive genomic method such as adhesive patch biopsy is also used to further label melanocytic lesions as benign or malignant to predict the need for biopsy study. The selection of these non- invasive techniques ultimately depends upon clinical utility, the cost versus advantage, and contending methodologies [11]. The principles and mechanisms were involved in skin cancer detection represent in Figure 2.
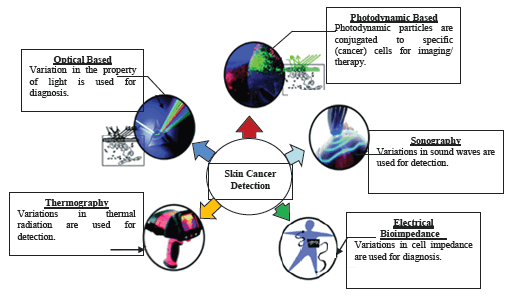
Figure 2: Principles and mechanisms for skin cancer detection
Biomarkers associated with cutaneous melanoma
The recognizable proof of biomarkers that can anticipate persistent advantages towards therapy is a focal disease research objective. B-RAF proto-oncogene (BRAF) mutations are a standard disease marker in response to RAF inhibitors. These cases develop disease progression after a variable timeframe and show primary resistance to BRAF inhibitors. Many studies have discussed the role of acquired genetic mutations, which affects the signalling pathways and, in turn, induces resistance to chemotherapy and targeted therapy in CM [18]. Currently, detecting the mechanisms responsible for BRAF and mitogen-activated protein kinase (MEK) inhibitor resistance is not a concern for clinicians; however, the development of non-invasive techniques for assessment of mutation status of a tumor will be more helpful [19]. A newly emerged liquid biopsy helps detect melanoma derived circulating cell-free DNA (cfDNA) in the plasma and acts as a promising blood-based biomarker in monitoring melanoma’s status. Several reports suggest that BRAF mutated melanoma detection through cfDNA before the commencement of treatment predicts BRAF kinase inhibitors’ response. Cases having high basal cfDNA levels are associated with a lower response rate and progression-free survival [19,20]. cfDNA is a predictive biomarker to detect tumor burden, an increase in cfDNA levels during treatment indicates disease progression and resistance acquired for inhibitors. Outstandingly, cfDNA helps detect mutations responsible for resistance to targeted BRAF therapies, and in the future, it can guide us for subsequent treatment strategies [19,20]. Immune checkpoint inhibitors have a low overall response rate (ORR). It was found that programmed cell death protein 1 (PD1) immunohistochemistry assays done on tumor specimens are not markers of choice to determine PD1 inhibitor treatment response due to the heterogeneity in clinical trials [21]. Many other predictive biomarkers are still under investigation. Recently, in humans, it was found that specific gut microbiota compositions can drive varying responses to immune checkpoint inhibitors [22,23]. This shows that the modulation of human gut microbiota composition might improve the immunotherapy response. Bioinformatics has yielded promising outcomes in identifying complex biological interactions in different pathways, having a specific immune system role. Computational models can mimic metabolic, biochemical, and immune-mediated interactions and describe how they are possibly engaged with melanoma advancement [1,24]. Hence, computational approaches may prompt identifying novel therapeutic targets and may shorten the drug discovery process.
Management and future prospective
Mostly, patients who are newly diagnosed with melanoma are at the primitive stage. For these cases, excision is the treatment of choice and it is the ultimate remedy [25]. Some cases relapse with the disseminated disease; however, 10% of melanoma cases are diagnosed at an advanced stage and are already metastatic. Amongst cases with stage IV tumours, one-third percent have brain involvement at the time of diagnosis are at a lower likelihood of sustaining the treatment response [26]. For such cases, revolutionization in therapeutic agents occurred since 2011. These agents are BRAF and MEK inhibitors and immune checkpoint inhibitors such as cytotoxic T lymphocyte-associated antigen 4antibodies (CTLA4) and PD1 antibodies. PD1 and CTLA4 antibodies (such as pembrolizumab, ipilimumab, and nivolumab), along with specific BRAF inhibitors (dabrafenib and vemurafenib) alone and/or blended with MEK inhibitors (cobimetinib and trametinib), have the promising outcome [27-33]. Immunotherapy and kinase inhibitors are considered promising therapy, while chemotherapy is considered a second- line treatment option [21]. PD1 and CTLA4 antibodies as therapeutic agents offer low response rates with a durable response [28,32,33]. In BRAF mutated melanoma, BRAF inhibitors and MEK inhibitors are used as a therapy. The blend has prompted high reaction rates (70%) with a quick response rate, along with an advantage of progression-free survival for a year [30-34].In some BRAF mutant melanoma cases, where BRAF inhibitor resistance has risen, nivolumab and pembrolizumab have shown to be effective [1,34,35]. The combination of PD1/CTLA4with targeted therapy must be considered as an experimental approach in recent clinical trials. Interferon-α treatment might be offered to patients with stage II and III melanoma as an adjuvant treatment, as these treatments increase infection-free survival time but disappoint due to toxicity. The consideration of patient attributes (such as lactate dehydrogenase and other biochemical parameters) with toxicity profile, along with comorbidities and individual patient inclinations, are focal components to be considered for cutting edge treatment strategy. Vital cooperation of patients in randomized clinical trials will be of great importance.
Decades after decades, clinicians’ and researchers’ valuable efforts helped develop new methodologies for diagnosing and managing cutaneous melanoma. With the increasing incidence of CM worldwide, efficient approaches are needed for the management of cutaneous melanoma. Eventually, the management of cutaneous melanoma depends on the individual patient staging and their response to the therapy.
Citation: Naik.P (2022), Cutaneous malignant melanoma: A review of confirmatory diagnosis and management J Can Sci Res 7:518
Received: 11-Apr-2022, Manuscript No. JCSR-22-7148; Editor assigned: 13-Apr-2022, Pre QC No. JCSR-22-7148 (PQ); Reviewed: 26-Apr-2022, QC No. JCSR-22-7148; Revised: 03-May-2022, Manuscript No. JCSR-22-7148 (R); Published: 11-May-2022 , DOI: 10.35248/2576-1447.22.7.518
Copyright: © 2022 Naik.P. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited credited.