Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2023)Volume 14, Issue 3
The Stevia rebaudiana (Bert.) leaves are natural low-calorie sweeteners used in many products, including foods, drinks, medicines, cosmetics, and more. This study aimed to use activated charcoal to remove color, purify stevioside from Stevia leaves, and utilize the chromatographic method to confirm its identity. We developed a novel eco-friendly method to decolorize and purify stevioside from Stevia leaf. The yield of stevioside was calculated by weighing the crystallized sample following freeze-drying. The average yield is 8.13%. Ultraviolet-Visible (UV-Vis) and Fourier Transform Infrared (FT-IR) spectroscopy were performed to confirm the identification. This purified sample showed a wavelength region of 4000–650 cm-1 in the FT-IR and a UV-Vis spectrum at 206.49 nm, which referred to it as a stevioside. In HPLC, the purified sample did an accuracy and precision test with standard stevioside, which gave a similar peak to the purified sample at the same retention time. According to High-Performance Liquid Chromatography (HPLC), the purity of stevioside is 98.12%. The pH effect on the decolourization of Stevia leaf extracts with activated charcoal was 5.5–8.0. In contrast to other commercially available methods, the above process can inexpensively purify stevioside from Stevia leaves.
Stevia rebaudiana; Stevioside; Decolorizing; FT-IR spectroscopy; HPLC
Stevia rebaudiana (Bertoni) is a tender perennial herb in Asteraceae family. It grows naturally in the soils of southern and central America that are slightly acidic [1,2]. The Italian-Swiss botanist Moises Santiago Bertoni first reported on the properties of this plant. Even though there are about 240 species in the genus Stevia, only S. rebaudiana has a sweet taste [3,4]. Indigenous people have used this plant's leaves for centuries in their treatments, sweeteners, and herbal tea [5,6]. Stevioside and rebaudioside-A are the primary two diterpene glycosides in Stevia's leaf and stem tissues and are responsible for their distinct sweetness [7,8]. Stevioside is 250-400 times sweeter than sucrose and contains no calories [9,10]. This sweetness compound passes through the digestive system without chemical breakdown, making it safe for people with diabetes and obesity [11,12]. It has many therapeutic benefits, including antihyperglycemic, anticancer, and antihypertensive properties, and it can also prevent dental caries [13-18]. It can also prevent bacteria and fungi from growing, and there have been no reports of adverse side effects so far [19]. It is used more frequently in the industry due to its inherent advantages. It was recognized as "Generally Recognized As Safe" (GRAS) in the USA in 2009 [20]. The Admissible Daily Intake (ADI) of stevioside was set by the World Health Organization (WHO) at 0–4 mg/kg of body weight [8,21]. At the beginning of the 1970, a group of Japanese researchers did a series of experiments to extract and purify sweeteners from Stevia leaves [22,23], which led to the first commercial use of stevioside from Stevia leaves. In recent years, many people in India, China, Brazil, Mexico, Africa, Canada, Europe, and the United States have been extracting stevioside for use in the industry [24,25]. Researchers have discovered that a combination of genetic and environmental factors and extraction techniques influences the enormous differences in stevioside contents in Stevia leaves around the world [26, 27]. Using the well-known HPLC method [28, 29], it is possible to completely separate stevioside and rebaudioside-A chromatograms. However, column conditioning takes a while, and because the retention time of the analysis varies so much, there is less method repeatability. In HPLC analysis, Solid Phase Extraction (SPE) is one of the techniques for sample preparation. SPE has the advantage of removing contaminants that could make the sample matrix less complicated and stop emulsions from forming in the extract solution. The SPE method has problems, like a higher cost [30]. Kennelly (2002) reported stevioside yields from dried leaves ranging from 5 to 22% [31]. Due to regulations and the high value of stevioside, an easy, cost-effective method is needed to obtain pure stevioside from Stevia leaves [32]. The activated charcoal method made a simple way to remove color and find stevioside. At different pH levels, stevioside examined the effect of pH on the decolourization of Stevia leaf extracts with activated carbon. This technique also improved to maximize the yield of stevioside. In this study, stevioside was extracted from Stevia leaf using activated charcoal, and its identity was confirmed using chromatographic techniques. This method of extracting stevioside from Stevia leaf is quick, cheap, and environmental friendly.
Plant material
From the Khargachari district in Bangladesh, we took 100 kg of dried S. rebaudiana leaves. A scientist identified Stevia at the Bangladesh National Herbarium, Mirpur, Dhaka. The leaves were then spread out on paper sheets and entirely dried in an oven at 65°C for three hours. The dried leaf is ground with a mortar and pestle to make a fine powder and stored at -4°C.
Reagents, standards, and instruments
We did the research at the Bangladesh Council of Scientific and Industrial Research (BCSIR) Industry Microbiology Laboratory in Dhaka, Bangladesh. Analytical grades of stevioside, acetonitrile, ethanol, sodium hydroxide (NaOH), hydrochloric acid (HCl), and activated charcoal (Mitsubishi Chemical, Japan) have been used in this study. Different instruments, i.e., the Milli-Q water purification system (Millipore, MA, USA), glass column, blender, rotary evaporator, freeze dryer, FT-IR spectrometer (PerkinElmer, USA), UV visible spectrometer (PerkinElmer, USA), HPLC (Hitachi, Japan), pH meter, etc., have been used.
Effect of pH on decolourization of Stevia leaf extracts with activated carbon and optimum contract time
For this experiment, Stevia fine powder was extracted with 70% ethanol. For each 50 g of Stevia fine powder, adjust the pH with 0.1 N HCl or NaOH solution, ranging from 5.5 to 10. The standard stevioside solution had a pH of 6.0. A spectrometer was used to measure the absorbance at OD 595 nm after mixing activated charcoal directly with Stevia leaf extract (1:3) and adjusting the pH to 6.0. After that, the 70% ethanol extract solution used different filtering times and took absorbance at different pH values ranging from 5.5 to 8.0 and 10, respectively.
Extraction and decolourization of stevioside with activated charcoal
The previous method used in this study was slightly modified [24]. Only fine powder was employed in the extraction method; coarse parts were discarded. In brief, 150 g of activated charcoal was appropriately mixed with 50 g of pine Stevia leaf powder and the added mixture with 750 mL of 70% ethanol solution. The mixture was then allowed to sit in a conical flask for 180 min. A glass column (8.5 cm × 6.5 cm) collected the mix once it had settled. The sample was passed through a glass column with 70% ethanol to remove the color compounds from the Stevia powder, and the fractions were collected in a container. The extracted fractions were collected and filtered through a 45 mm filter paper. A rotary evaporator at 45°C evaporated the solvent after creating a colourless solution. The residual solvent, which was only a tiny amount of water, was dried with a freeze-dryer. The crystalline, colourless substance was weighed and kept in a freezer at -4°C for further analysis.
Measurement of UV-visible spectra
Light absorption by a colourless crystal substance is the basis for UV-vis. While using a spectrophotometer to identify a stevioside by UV-vis, the instrument was calibrated first. The cuvette is shown light with wavelengths between 190 nm and 800 nm, and both the emission and absorption spectra are recorded.
Measurement of FT-IR spectra
The colourless crystal material was scanned using an FT-IR spectrophotometer with a Deuterated Triglycine Sulfate (DTGS) detector and a germanium beam splitter. Thirty-two scans were undertaken on FT-IR spectra in the wavelength range of 4000-650 cm-1 with a resolution of 4 cm-1. All spectra were set up using the background spectrum of air as a standard. After each scan, a new reference air background spectrum was recorded. At each data point, the absorbance values from these spectra were recorded.
HPLC analysis
Sample preparation is an integral part of the HPLC for isolating and purifying stevioside. We prepared a stock solution of standard stevioside. In volumetric flasks, 1 mg of standard stevioside was dissolved in 2 mL of acetonitrile: water (80:20) mixture, making a 500 ppm solution. From this stock solution, finally make solutions with 100, 200, 300, and 400 ppm concentrations. These were used to make the standard curve. The colourless crystal substance was dissolved in an acetonitrile: water (80:20) mixture. The isocratic mobile phase was comprised of acetonitrile and water (80:20) v/v, and the crystal colourless substance was analyzed using HPLC coupled with the 5 µm refractive index detector and NH2 column (125 × 4.6 mm) [33]. Additionally, employing the same system, connected to a Diode-Array Detector (HPLC-DAD) (Hitachi, Japan), extracts were filtered through nylon filters with pore sizes of 0.22 µm before being diluted tenfold with water:acetonitrile (2:8, v/v) solvents. A Hydrophilic Interaction Liquid Chromatography (HILIC) column (9.4 × 250 mm, 5 µm) was used for the separations (ZORBAX, USA). According to the isocratic program: 0-30 min, 80% B, Milli-Q water, and acetonitrile were provided as aqueous (A) and organic (B) mobile phases. The solvents for the mobile phase were filtered, and each was degassed on a sonicator before mixing them. The column was thermostated at 25°C; the flow rate was set at 1 mL min−1, and set the injection volume at 20 µL. The diode-array detector worked in the 190 to 350 nm wavelength range. By comparing the retention time and UV–visible spectra to the reference standard, we confirmed the presence of stevioside. HPLC did the reading at a 210 nm wavelength. In the same program, HPLC also ran a standard stevioside concurrently. Stevioside analysis techniques come in many forms. The HPLC method is the foundation of most of the quantitative stevioside methods.
Decolourization of Stevia leaf extracts with activated charcoal
The extraction and purification method of stevioside has one step that involves the removal of impurities and color compounds from Stevia leaf powder by activated charcoal. It is a type of carbon processed with tiny, low-volume pores that increase the surface area available for impurity adsorption from Stevia fine powder. One gram of activated charcoal has a surface area of more than 500 m2 (5,400 sqft) because of its high level of microporosity [33]. Large organic molecules are bound to the surface of activated carbon through a process known as adsorption. The adsorption capacity of a Stevia powder on an activated carbon surface depends on the substance's concentration, temperature, and the polarity of the essence. The activated charcoal did not remove all polar compounds but removed the non-polar compounds. Plant pigments, carotenoids, chlorophyll, by-products, tannins, phenolics, poly-aromatics, and products of the Maillard reaction between sugar and amino acids are among the substances that give the extracted color throughout the Stevia extraction process. Adsorption binds impurities on the surface of the activated charcoal filter rather than absorbing them. Thus, stevioside is purified of its colours. The Stevia leaves were extracted from a glass column using activated charcoal and 70% ethanol. The discoloured stevioside was then recovered. After purification, a total of 4.065 g of stevioside was obtained from 50 g of fine Stevia leaf powder. The percentage yield is 8.13% (Figure 1). Color chemicals are adsorbed onto activated charcoal, which serves as the adsorbent. When activated charcoal is treated with 70% ethanol, the colorants in Stevia leaf extracts gradually adsorb onto the adsorbent. In combination with activated charcoal and at a particular pH level, Stevia leaf extracts contract with time. All color pigments are removed by charcoal adsorption. In the end, white, pure stevioside was obtained.
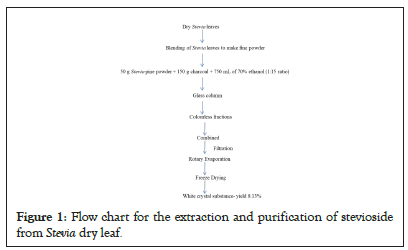
Figure 1: Flow chart for the extraction and purification of stevioside from Stevia dry leaf.
Analysis of stevioside using UV-visible spectra
Generally, the UV and visible spectral bands of compounds are large. Nonetheless, they are sufficient for quantitative assays and valuable as an alternate means of detecting many compounds [34]. The radiation from typical hot solids consists of several wavelengths and depends primarily on the temperature of the solid. The energy released at each given wavelength is predictable from the principle of chance [30,35,36]. The spectrum in Figure 2 reveals that a peak for pure stevioside is observed at wavelength 206.49 nm. In this context, absorbance as a function of wavelength is used to represent the UV-vis spectrum graphically.
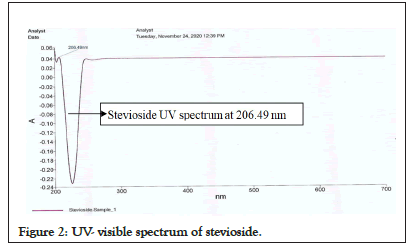
Figure 2: UV- visible spectrum of stevioside.
Analysis of stevioside using FT-IR spectra
FT-IR spectra of stevioside are presented in Figure 3. The broad and intense absorption at 3313.28 cm-1 corresponded to the stretching vibration of the OH bond (-OH stretching) and was associated with the presence of the hydrogen bond. Absorption at 2932 cm-1 was characteristic of the extension–CH sp3 bond. The maximum intensity peak at 1619.79 cm-1 corresponded to the stretching vibration –C=O bond. At 1401 and 1347.7 cm-1, the bending vibration observed -CH bond. Furthermore, high-intensity peaks at 1050 cm-1 corresponded to C-O derived from stevioside and were characteristic absorption bands of the glycosidic bond. Finally, it revealed that the peaks at 928.59 cm-1 and 864.74 cm-1 were the bending vibrations of the =CH and =CH2 bonds, respectively [25]. The absorption and intensity values in the infrared frequency range are not identical due to interactions between molecules in the colourless crystal substance and infrared radiation [26]. The calibration of FT-IR on standard stevioside was achieved by matching significant data at the fingerprint frequency region to the stevioside functional group, which led to the recognition of FT-IR as a fingerprint technique [27].
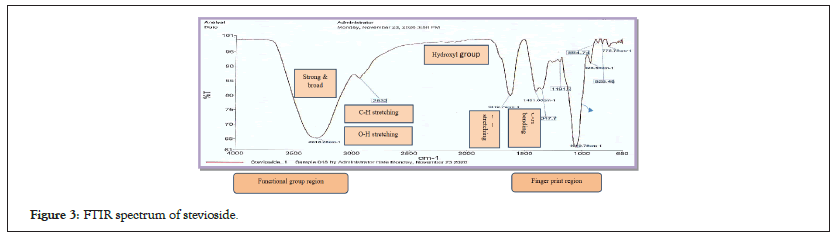
Figure 3: FTIR spectrum of stevioside.
Analysis of stevioside by HPLC
Five concentrations (100, 200, 300, 400, and 500 ppm) of stevioside and colourless crystal substance were analyzed by HPLC. Peaks for the standard stevioside and colourless crystal substance were observed at retention times of around 10.5 min (Figure 4). HPLC analysis reveals that the purity is 98.12% compared to the standard stevioside. Using an amino column and the solvent system acetonitrile: water (80:20) in HPLC has also separated stevioside [37]. For the measurement of stevioside, HPLC set a flow rate of 1 mL/min and a wavelength of 210 nm [38]. In the present investigation, the activated charcoal method has been used for extracting stevioside from Stevia leaves. Compared to previously reported methods, this method has the potential to remove stevioside [39]. The benefits of traditional extraction over contemporary extraction techniques include their general industrial use and higher efficiency. Despite these advantages, this method has significant drawbacks, including a lengthy process and a high solvent requirement. The food and pharmaceutical industries greatly value stevioside isolation and purification as a natural sweetener with low calories.
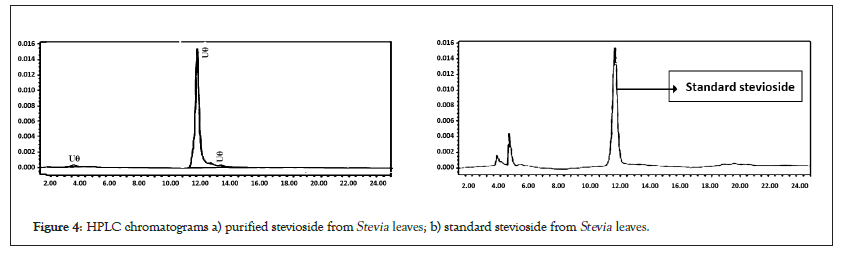
Figure 4: HPLC chromatograms a) purified stevioside from Stevia leaves; b) standard stevioside from Stevia leaves.
Effect of pH value on the activated charcoal adsorption process in Stevia leaf extracts
The amounts of adsorbed activated charcoal for different pH values and times of contraction during the adsorption process are given in Figure 5. The experiment shows that the pH value significantly influences adsorption capacity, i.e., the amount of activated charcoal. The pH value of 70% ethanol Stevia leaf extract significantly affects decolourization with activated charcoal. With increasing pH, absorbance values increased initially, but after adding activated charcoal, absorbance values decreased gradually. The decolourization method was complete when the absorbance value for all pH (from 5.5 to 8.0) values in the Stevia leaf extract was zero (0) after around 180 min. This analysis indicates that no color compounds were present in the Stevia leaf extract at this condition. The behaviour of this extract in this experiment was different because the structure of the chemical compounds in Stevia extracts unravels at a higher pH (pH 8). The Stevia leaf extract absorbance at pH 10 was a very high 0.75. Within 10 min of adding the charcoal, the absorbance started to decline before gradually rising again. At pH 10, the Stevia extract solution achieved the endpoint. This disparity indicated the endpoint of the pH values and the contracted time. Stevia leaf extracts found the highest impurity adsorption capabilities in with a pH value of 5.5 to 8.0, where a pH value of 10.0 was substantially lower. Figure 5 shows that the concentration of contaminants with coloured compounds in the Stevia leaf extract in 70% ethanol rapidly decreases. The process can be effectively split into three regions: rapid initial adsorption (pH value 5.5), followed by a milder (pH value 6.0) and more progressive (pH value 6.5) reduction in color compounds, which eventually achieves the equilibrium condition. In Figure 5, it is also demonstrated that, except during the contract period, the color concentration in the liquid phase for a pH value of 10 is roughly equal for all contact times. We compared the results obtained using the same adsorption experiment technique but without altering the pH value before adsorption to calculate decolourization efficiency for all pH values except pH 10. The surface charge of activated charcoal and interactions between activated carbon and coloured Stevia extract compounds, which dissociate on coloured anion and sodium ions, can explain why the pH value is at its maximum at pH 8.0. The surface of the activated charcoal becomes positively charged and attracts the coloured anion via strong electrostatic forces if the pH value is lower than the charcoal's point of zero charges. These attractions are more robust when the pH value is lower than other pH values. According to earlier reports, commercially available activated charcoal has pH values that range from 6.50 to 7.33 [26,27,34]. These are given to the theory that solid adsorption at pH=5.5 may be caused by strong electrostatic interactions between the anions in Stevia extract and the surface of activated charcoal. Under various conditions (pH and time duration), the adsorption of coloured compounds on activated charcoal was investigated. Adsorption reached equilibrium after 180 min. The pH had a significant impact on adsorption. The difference in adsorption caused by pH values may be due to the ionization of contaminants in Stevia extract into cationic and anionic species, as well as the corresponding positively and negatively charged carbon surfaces. These processes occurred at low and high pH values. A pure 70% ethanol extract of Stevia leaves usually has a pH of 6.0. From Figure 5, it can be observed that, with increasing pH, the absorbance value increased before charcoal addition, but for all pH values, it decreased with time after charcoal addition. Only a pH 10 Stevia leaf extract demonstrated the opposite behaviour. However, this experiment rejects this principle and reveals the opposite behaviour at a high pH value (pH 10) and a brief period of contraction time.

Figure 5: Effect of pH values on the Stevia leaf extract in 70% ethanol after an appropriate time of adsorption (pH = 5.5; pH = 6.0).
In this research, we used activated charcoal advantageously to remove the impurities and color compounds from the residue, leaving behind the stevioside. Stevia leaves were extracted with 70% ethanol, and stevioside was purified by partitioning stevioside between solvent and activated charcoal. After the completion of drying, 4.065 g of stevioside was obtained. The percentage yield is 8.13%. The main aim of this work was to optimize a simple and efficient extraction method for recovering stevioside from Stevia leaves. Thus, following the principles of green chemistry, ethanol was used as the extracting solvent, followed by activated charcoal to obtain stevioside. Preliminary experiments were conducted to select the optimum separation conditions, and the best results in terms of activated charcoal and reproducibility were obtained using 70% ethanol. A solvent system (n-hexane: n-butanol: water) at an optimized volume (ratio of 1.5:3.5:5) was selected for the High-speed counter current chromatography (HSCCC) separation. The lower phase was employed as the mobile phase in the head-to-tail elution mode. In a single procedure, 200 mg of the crude extract yielded 54 mg of 98.3% pure stevioside. Several other workers supported the results using a mixture of chloroform and methanol with gradually increasing polarity to purify stevioside through the column chromatography technique [29]. Pure stevioside crystals were separated by adding a small amount of methanol to the fractions containing stevioside. These findings are consistent with other researchers' reports [30,39]. The filtrate was concentrated under a vacuum and sprayed dry. The extract was dissolved in methanol at ambient temperatures to precipitate stevioside. Recrystallization with methanol resulted in pure stevioside [30]. To quantify stevioside, acetonitrile, and water as the mobile phase at a flow rate of 1 mL/min and a wavelength of 210 nm have also been used.
In the present investigation, the activated charcoal extraction method has been used for extracting stevioside from Stevia leaves. The method can remove more sample mass than the other methods. Also, wide industrial application and better efficiency are advantages of conventional extraction over modern extraction methods. While this procedure has some benefits, it also has some drawbacks, including time consumption and the need for a significant volume of solvent. Stevioside, isolated through such studies, is very useful in the food and pharmaceutical industries as a low-calorie natural sweetener. Finally, a glass column used 70% ethanol-activated charcoal with a pH of 5.5 to maximize stevioside extraction from Stevia leaf extract. Decolourization and purification of stevioside from Stevia leaf extracts by solvent extraction with activated charcoal could be a reliable, rapid, and efficient technique. FT-IR spectroscopy, combined with UV-vis and HPLC analysis, could confirm stevioside in Stevia leaf. However, most stevioside purification methods require expensive operating conditions and sophisticated equipment. This study provides extraction efficiency that is easy to use, relatively inexpensive, and environmental friendly.
This study was funded by the R&D project of the Institute of Food Science and Technology (IFST), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka. The authors are grateful to IFST and BCSIR authority as well.
This research is being funded as part of BCSIR R&D project.
MNIB designed the study, performed the experiments with KAAS, and analyzed the data. MN, SA, MAH, MKUS, MMH, MASM, and MAAS gave suggestions, and wrote the introduction. All authors contributed to the drafting of the manuscript.
Citation: Bhuiyan MNI, Shamima KAA, Nahid M, Afrin S, Hoque MA, Haque MM, et al (2023) Decolorizing Stevia rebaudiana (Bert.) Leaf Extracts with Activated Charcoal and Qualitative Analysis of Stevioside Using Chromatographic Methods, J Chromatogr Sep Tech. 14:513.
Received: 01-Apr-2023, Manuscript No. JCGST-23-22800; Editor assigned: 04-Apr-2023, Pre QC No. JCGST-23-22800 (PQ); Reviewed: 24-Apr-2023, QC No. JCGST-23-22800; Revised: 03-May-2023, Manuscript No. JCGST-23-22800 (R); Published: 12-May-2023 , DOI: 10.35248/2157-7064.23.14.513
Copyright: © 2023 Bhuiyan MNI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.