Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research - (2022)Volume 12, Issue 5
Tire Rubber (TR) is a material that despite being recalcitrant can be biodegraded. Biodegradation is more effective after detoxification, and includes biodeterioration, depolymerization, and assimilation of the material by microorganisms. The potential for TR biodegradation in filamentous fungi isolated from deteriorated tires was explored. Its growth was evaluated on Radha agar with ground tire rubber (GTR) at concentrations (w/v) of 0.6, 1.5, 15, 45, 100% and in liquid media at 0.8%. The best growth was obtained at the 15% concentration, possibly due to a balance between the carbon source and the toxicity of the material. Six morphotypes grew significantly at the 100% concentration, and four significantly acidified the liquid media, suggesting the ability to solubilize metals. These ten (10) morphotypes that showed biodeterioration potential by growing on the polymer were identified by their phenotype. The ability to acidify the liquid media of Curvularia sp. 2 and its growth in 100% TR agar, made it of special interest, along with four Trichoderma spp. morphotypes, whose growth in all concentrations and previous reports of degradation of other polymers suggest great potential to depolymerize and assimilate TR.
Biodegradation; Biodeterioration; Depolymerization; Assimilation; Detoxification
Billions of waste tires are discarded in landfills throughout the world each year, creating risks to human health and the environment [1]. While the landfills for the safe disposal of rubber waste reach their limit, their indiscriminate disposal causes water and soil contamination. Likewise, the burning of rubber waste generates a large amount of heat and smoke that influence global warming and air pollution [2].
The environmental risks associated with waste tire rubber and its disposal are due both to its complex three-dimensional structure, as well as to its composition and chemical additives that preclude their direct recycling [3]; in addition to being hard to biodegrade [2]. The aforementioned factors contribute to the accumulation of the material.
Tires, usually, are made by mixing natural and synthetic rubber [4]. Different compounds are added to this mixture to increase strength and abrasion resistance [5]; additionally, this mixture is vulcanized through a thermochemical process that involves complex reactions between rubber polymers, sulfur, and additives [4-6]. Although there are other vulcanization methods, the thermochemical is the most used for tire manufacturing [7]. Despite its complex composition, tire rubber can be biodegraded [2]. Biodegradation of polymeric materials includes: biodeterioration (initial changes in the material by microorganisms growing on its surface), depolymerization (enzymatic breakdown of the polymer into oligomers, dimers, and monomers), and assimilation (entry of compounds into the cell for metabolization) [8]. Microorganisms such as fungi and bacteria can degrade natural and synthetic rubber and use it as a carbon source [9]. Some fungi, particularly white and brown rot fungi such as Ceriporiopsis subvermispora, are capable of devulcanizing rubber [10], a process that involves the desulfurization of the material [11], meaning the breaking of the sulfur-sulfur and sulfur-carbon bonds [4] via enzymes [5]. This biochemical process can promote the degradation of rubber materials. However, many microorganisms are sensitive to additives present in this substrate [12], such as zinc salts and oxides [2], heavy metals such as Mn, Fe, Co, Ni, Cu , Zn, Cd, and Pb [13] or polycyclic aromatic hydrocarbons (PAHs) [14]. These additives can prevent or limit the growth of organisms and inhibit their ability to biodegrade [7]. Fungal species such as Recinicium bicolor can degrade aromatic compounds found in rubber and have been successfully used to detoxify the material [2,15]. The ability of these microorganisms to degrade polymers depends on the presence of extracellular enzymes [16]. Nayanashree and Thippeswamy [17] report that the enzymes laccase and manganese peroxidase are responsible for the degradation of natural rubber. Both enzymes are frequently found in the Fungi kingdom [18,19]. According to Shah et al. [1], De Vries [20] was a pioneer in examining the possible decomposition of rubber by fungi. A decade later, Kalinenko [21] reported isolates of the genera Aspergillus and Penicillium as rubber degraders. Another species, Paecilomyces variotii strain SFA-25, was reported by Zeb [22] as a degrader of vulcanized rubber.
Thus, there are reports of fungi capable of biodegrading tire rubber and carrying out the two previous processes that promote degradation: detoxification and devulcanization. This suggests that fungi have potential in tire rubber degradation, at different stages and through different processes. Taking into account what was established above, the potential to degrade tire rubber of filamentous fungi isolated from samples of deteriorated tires on the Bogotá D.C-Silvania-Cundinamarca Road, Colombia, was evaluated.
Sampling place
Five sampling sites were selected on the Bogotá, D.C-Silvania road, and department of Cundinamarca, Colombia. The places were selected considering the presence of at least three abandoned tires with evident deterioration (Figure 1A). Three tires were selected from each site and three pieces were removed from each using a sharp knife disinfected with 5% (v/v) hypochlorite. Additionally, with a garden shovel (disinfected with 5% v/v hypochlorite), a 40 g sample was taken from the soil in contact with the tires (both types of samples were stored in Ziploc® resealable bags). A total of 45 pieces of tires and 15 soil samples were analyzed in the Laboratory of Environmental and Soil Microbiology of the Pontifical Xavierian University, Bogota campus.

Figure 1A: Deteriorated tires with fungal growth and soil.
The sampling sites were: Abandoned Gallera, municipality of Soacha, Cundinamarca (N 04°32.492' W 074°15.041'). Outskirts of Bogotá, Alto de Rosas (N 04°32.643’ W 074°17.174’). Montallantas, route to Silvania, Cundinamarca (N 04°27.607’ W 074°23.086’). Brio “El Vergel” service station, route to Silvania, Cundinamarca (N 04°26.935’ W 074°22.754’); Montallantas, Azafranal, Silvania, Cundinamarca (N 04°25.086' W 074°23.338' (Figures 1A and 1B).

Figure 1B: Deteriorated tires with fungal growth and soil, collected on the Bogotá, D.C- Silvania, Cundinamarca route.
Isolation of fungal morphotypes
For the isolation of fungi, pieces of tires in Figure 2A of approximately 12 cm2 were placed in humid chambers, at room temperature (approx. 14°C), for four weeks. Then, four 1 cm2 pieces were cut from each sample and inoculated (two repetitions per sample) on Petri dishes with potato dextrose agar (PDA) (glucose 15 g L-1, agar 1.5% m/v of, potato infusion 200 mL L-1 and chloramphenicol (clo), 50 mg L-1) (Figure 2A) and on rose bengal agar (OXOID, Ref. CM1149), incubating for 10 days at (30 ± 1) °C. For the soil samples, serial 10 base dilutions were made in NaCl 0.85% (m/v) up to 10-5 and the 10-3 and 10-5 dilutions were inoculated on PDA+ clo agar (two repetitions per sample).
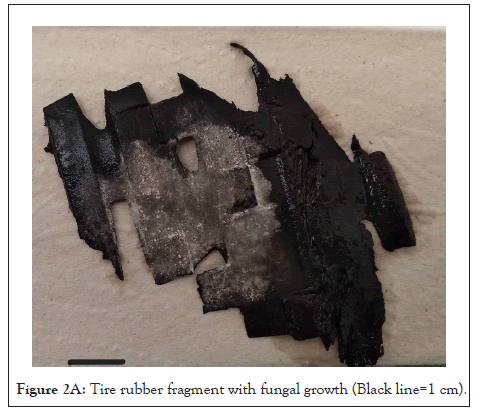
Figure 2A: Tire rubber fragment with fungal growth (Black line=1 cm).
On the other hand, 50 ml of minimum salt broth (K2HPO4 8g L-1, KH2PO4 1g L-1, (NH4)2SO4 0.5g L-1, MgSO4 × 7H2O 0.2g L-1, NaCl 0.1 g L-1, Ca(NO3)2 0.1 g L-1, CaCl2 × 2H2O 20 mg L-1, FeSO4 × 7H2O 20 mg L-1, Na2MoO4 × H2O 0.5 mg L-1, MnSO4 0.5 mg L-1) [23] supplemented with 0.1% (m/v) glucose, were placed in a 100 mL Erlenmeyer flask and 4 mL of the 10-3 dilution of each soil sample were added ( two repetitions per dilution, n=15). Subsequently, in an analytical balance (ScoutPro OHAUS), we weighed (0.25 ± 0.01) g of tire pieces that were sterilized in an autoclave and added them, as bait, to the broths. They were incubated in a New Brunswick ™ Innova 44 orbital rotator at 120 rpm, at (25.0 ± 0.1) °C for two weeks (adapted from Tsuchii and Tokiwa [24]). After the incubation time, the pieces were gently washed in distilled water, and they were used as an inoculum in PDA+ clo media.
The greatest possible number of fungal morphotypes was isolated by central puncture technique in PDA+ clo, from the growth obtained in the 210-culture media (180 from the humid chamber experiment, and 30 from the orbital rotator experiment). On the other hand, the most representative morphotypes (those that had a greater radial growth or that appeared more frequently) were isolated from the 60-culture media from the serial dilutions of soil samples (Figures 2A and 2B).
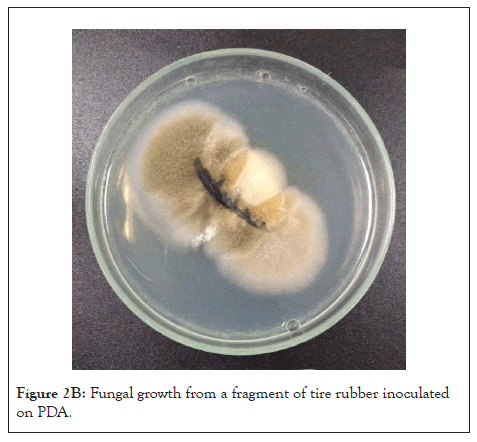
Figure 2B: Fungal growth from a fragment of tire rubber inoculated on PDA.
Selection of isolated morphotypes and measurement of growth at different concentrations of ground tire rubber (GTR)
The morphotypes were grouped according to their sampling location and their morphology. The most representative morphotypes of each group were selected, eliminating similar ones. The growth of the selected fungi was tested in GTR agar (rubber particles did not exceed the (5 ± 1) mm). In order to prepare the TR medium, Radha agar was prepared (CuSO4 1.5mM L-1, KH2PO4 2g L-1, NH4Cl 0.05g L-1, MgSO4 × H2O 0.5g L-1, CaCl2 × 2H2O 0.1g L-1, 10mL L-1 of elements solution trace (MnSO4 0.5 g L-1, FeSO4 × 7H2O 0.1g L-1, ZnSO4 × 7H2O 0.1gL-1)) [25] with 0.6% (m/v) GTR, 0.5% (m/v) glucose and 2% (m/v) agar. In duplicate, the media were inoculated with a 0.7 cm diameter disk of PDA + clo medium with the mycelial growth of each morphotype. The media were incubated at (30 ± 1) °C (VELP™ Scientifica FOC 225I) for two weeks, recording their radial growth every five days.
From the morphotypes that grew in GTR agar at 0.6% (m/v), agar discs with fungal mycelium were taken and the previous procedure was followed, inoculating them in GTR agar at 1.5%, 15% and 45% (m/v) (Figure 2B). The morphotypes that grew in the highest concentration of GTR agar were inoculated in Petri dishes with 7 g of sterile GTR and moistened with 7 mL of Radha medium (100% GTR medium). The identification of the morphotypes of interest was carried out through macroscopic and microscopic description, using lactophenol blue staining and Barnett and Hunter taxonomic keys [26].
Evaluation of the degrading potential of the selected morphotypes
100 mL Erlenmeyer flasks were filled with (200.00 ± 0.01) mg of GTR, weighed on a Mettler™ Toledo xp26p balance, with glucose 0.5% (m/v) and 25 mL of Radha broth, sterilized in an autoclave and inoculated with a 0.7 cm disc of each morphotype selected and previously grown on 0.6% (m/v) GTR agar (two repetitions per morphotype) (adapted from Tsuchii et al. [27]). The broth without inoculum was used as a negative control. Cultures were kept for eight weeks [17] under constant agitation, in an Innova™ 44 orbital rotator at 110 rpm and at (30.0 ± 0.1) °C [28]. After incubation (Figure 2C), the GTR was separated from the Radhamedium, using a coffee filter (Stilocafé #8). The filtered medium was collected in 50 mL Falcon™ tubes and its pH was determined in an Okaton™ pH meter. To separate the biomass from the GTR, the solid particles filtered were placed in 100 mL Erlenmeyer flasks with 20 mL of sterile distilled water. Then, the flasks were subjected to agitation in a Velp Scientifica™ ZX Classic vortex at 3000 rpm for 20 seconds. The polymer was centrifuged at 4000 rpm for 10 minutes in a PowerSpin™ CENTRIFUGE UNICO, the supernatant (water, mycelium and some tire particles) was discarded and the precipitate was sterilized to eliminate traces of fungal cells. Then, the GTR was dried for 36 hours in a Memmert™ UF55 oven at (45.0 ± 0.1)°C.
Figure 2C: Fungal growth on GTR agar. D. Fungal growth in Radha+GTR broth.
Each sample was analyzed by Fourier Transform Infrared Spectrometry (FTIR), in a Shimadzu™ MIRacle 10, coupled with an ATR (Attenuated Total Reflection) cell. The results of the treatments were compared with those of the control. Additionally, the samples were observed by scanning electron microscopy (SEM), in a Jeol™ JSM 6490LV, using a voltage of 10 kV and obtaining the signals of the secondary electron detector (SEs). Images were obtained at magnifications between 450 and 5000X. Before observation, the slides were plated with gold in a Denton™ Vacuum Desk IV metallizer [29]. Tire rubber samples in which the growth of microorganisms was evident were treated with Calcofluor White [30] and observed with an Olympus™ FV1000 confocal microscope (Figures 2C and 2D).

Figure 2D:Fungal growth in Radha+GTR broth.
Selection of morphotypes and growth measurement at different concentrations of GTR
Approximately 300 morphotypes were isolated from the collected soil and deteriorated tire samples, whose number decreased after the selection processes at different concentrations of GTR agar. The concentration from which more morphotypes were isolated was 0.6 m/v of GTR (Table 1). It is important to highlight that all the morphotypes that grew at a concentration of 100% GTR medium, were obtained from isolations made from the deteriorated tire pieces that were incubated in a humid chamber.
Radial growth of the morphotypes that grew in the 100% GTR medium was evaluated. 17 out of 21 morphotypes were analyzed (growth in multiple satellite colonies was not measured). Two factorial ANOVAs were performed (Design-Expert® version 9 software) whose independent categorical variables were GTR concentration (0.6, 1.5, 15, 45, and 100%) and morphotype (17 analyzed) for both; one used radial growth as a response variable (mm) and the other used the productivity (defined as maximum growth in mm/day in which it was evidenced). The F-statistic for the model was 8.2 (p-value<0.0001), for the concentration variable the value was 19.99 (p-value<0.0001) and for the morphotype variable, 5.25 (p value<0.0001). The model had a R2 of 0.7193, indicating the interdependence between the variables.
On the other hand, productivity was used as a response variable as it includes time as a factor in the analysis. The F-statistic for the model was 7.32 (p-value<0.0001), for the concentration variable the value was 17.19 (p value<0.0001) and for the morphotype variable, 4.85 (p-value<0.0001). The model had a R2 of 0.6957, indicating the interdependence between the variables.
In both statistical analyses, the best results for the growth and productivity variables occurred at a concentration of 15% (m/v), for all the morphotypes evaluated. The morphotype that obtained the highest growth and productivity values in all concentrations was Trichoderma sp.4.
The morphotypes whose values were statistically significant for both response variables were phenotypically identified as Trichoderma sp., Trichoderma sp. and Aspergillus sp., isolated from sampling site #2; Trichoderma sp. 3 and Curvularia sp. isolated from sampling site #3; Curvularia sp. and Mucor sp. from sampling site #4 and Mucor sp., Trichoderma sp. 4 along with an unidentified isolate, isolated at sampling site #5. For sampling site #1, fungi were not selected since the isolates did not meet the selection criteria. Some of the isolated fungi are shown in Figures 3A-3F.
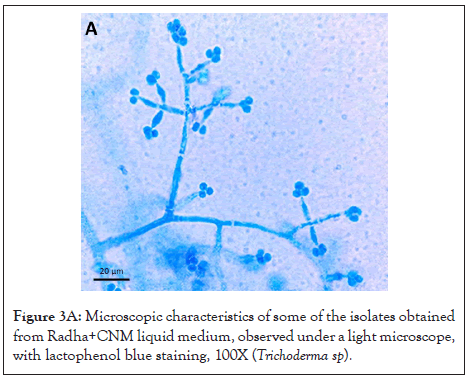
Figure 3A: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium, observed under a light microscope, with lactophenol blue staining, 100X (Trichoderma sp).
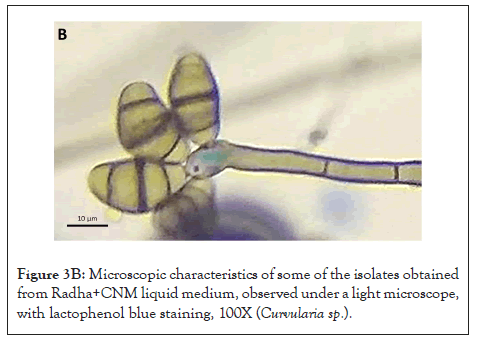
Figure 3B: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium, observed under a light microscope, with lactophenol blue staining, 100X (Curvularia sp.).
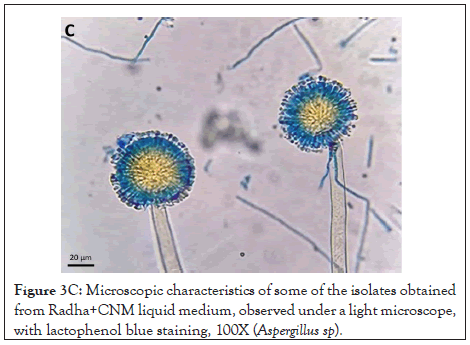
Figure 3C: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium, observed under a light microscope, with lactophenol blue staining, 100X (Aspergillus sp).
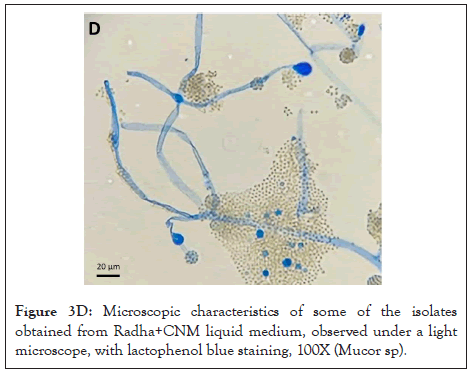
Figure 3D: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium, observed under a light microscope, with lactophenol blue staining, 100X (Mucor sp).
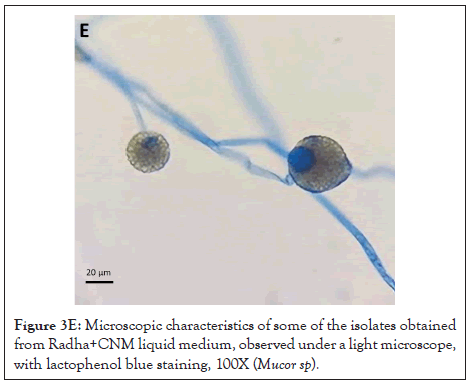
Figure 3E: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium, observed under a light microscope, with lactophenol blue staining, 100X (Mucor sp).
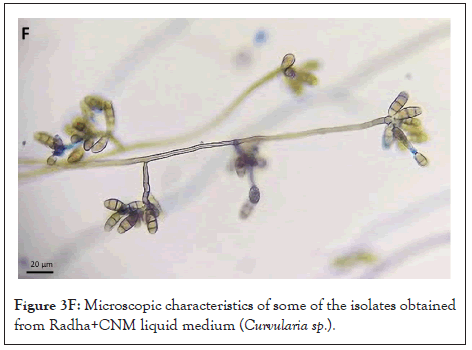
Figure 3F: Microscopic characteristics of some of the isolates obtained from Radha+CNM liquid medium (Curvularia sp.).
Evaluation of the degrading potential of the selected morphotypes
The evaluation of the degrading potential of the selected morphotypes was carried out through variables such as radial growth, acidification of the culture medium, evaluation of functional chemical groups by FTIR and confocal laser scanning microscopy.
Regarding the pH values obtained in the Radha medium with the GTR experiment, although all isolates generated a decrease in pH, Curvularia sp.1 (pH=2.38 ± 0.01), Curvularia sp. 2 (pH=3.00 ± 0.01), Mucor sp. 1 (pH=3.15 ± 0.01) and Aspergillus sp.1 (pH=2.43 ± 0.01), were the ones that generated greater acidification of the medium compared to the control (pH of the culture medium without inoculum=4.20 ± 0.01) (Table 2).
Curvularia sp. 2 was able to tolerate concentrations of up to 100% GTR medium, although it did not show significant growth compared to the other morphotypes evaluated. On the other hand, Curvularia sp. 1 grew, albeit with limited growth, up to a concentration of 45% GTR (m/v) (Table 2). Aspergillus sp. 1 also grew at all concentrations, however, due to its growth in numerous satellite colonies at 0.6, 1.5, 15 and 45% (m/v), its diameter could not be measured and was not statistically analyzed. At the 100% GTR medium, the growth was reduced (Table 2).
Mucor sp. 1 grew up to 8.4 cm at a concentration of 100% (m/v) while Mucor sp. 2 did not grow at concentrations above 0.6% (m/v). Finally, all isolates of Trichoderma spp. and the unidentified morphotype grew at all concentrations of GTR (Table 2).
Regarding the FTIR-ATR analyses, Figure 4A shows the spectra of the control sample (tire without biological treatment black line) and after exposure to fungi (red line). Figure 4A exhibits the unidentified morphotype, while Figures 4B and 4C show Trichoderma sp. 2 and sp. 3, respectively. Bands observed above 3400 cm-1 correspond to a signal of OH bonds [31]. A change in this signal between 600 and 1000 cm-1 is displayed, corresponding to the stretching of the C-O bond.
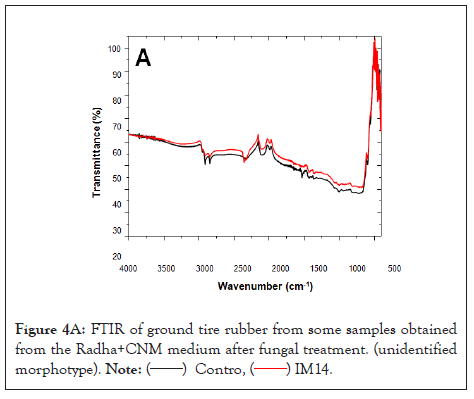
Figure 4A: FTIR of ground tire rubber from some samples obtained from the Radha+CNM medium after fungal treatment. (unidentified morphotype).
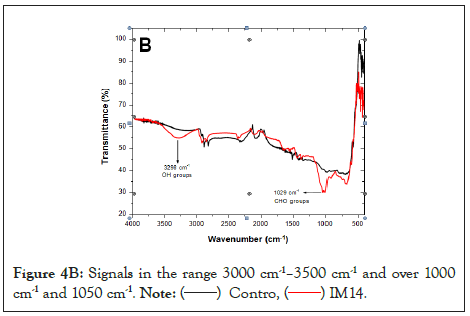
Figure 4B: Signals in the range 3000 cm-1–3500 cm-1 and over 1000 cm-1 and 1050 cm-1.
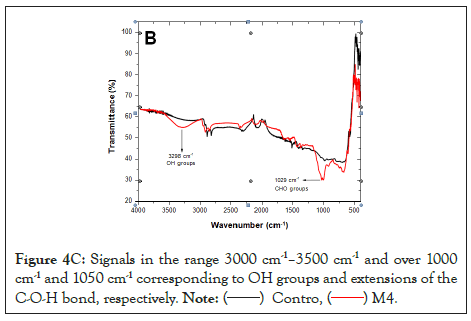
Figure 4C: Signals in the range 3000 cm-1–3500 cm-1 and over 1000 cm-1 and 1050 cm-1 corresponding to OH groups and extensions of the C-O-H bond, respectively.
Finally, the growth of fungi on the tire samples was observed using confocal microscopy and SEM. Figures 5 and 6 show examples of mycelial adhesion to the substrate.
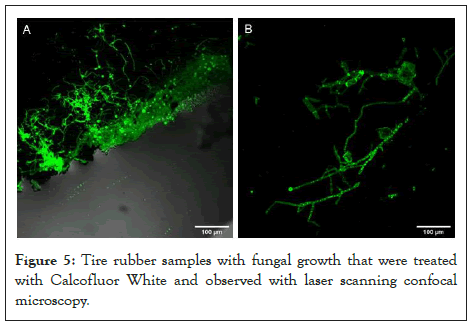
Figure 5: Tire rubber samples with fungal growth that were treated
with Calcofluor White and observed with laser scanning confocal
microscopy.
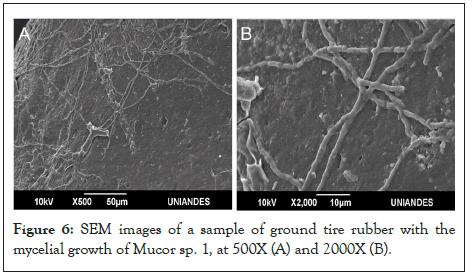
Figure 6: SEM images of a sample of ground tire rubber with the mycelial growth of Mucor sp. 1, at 500X (A) and 2000X (B).
The results obtained show the growth of different morphotypes of filamentous fungi on GTR agar at different concentrations. According to Lucas et al. [8], the growth of microorganisms in a "polymer agar" can indicate its consumption by the inoculated species. It could be that some morphotypes used their mycelial reservoirs as a carbon source instead of the polymer to grow. However, 90% of the isolated morphotypes did not grow at the lowest concentration of GTR agar; and only 21 morphotypes grew on 100% GTR. In addition, the interdependence between the concentration and growth variables was statistically demonstrated, which suggests the use of tire rubber as a source of carbon.
The growth of numerous morphotypes was inhibited by the presence GTR, even at minimal concentrations (0.6% m/v) and increasing GTR concentration reduced the growth of several morphotypes (Table 1). Thus, we will mention some possibilities on how the identified species were able to grow in this medium, despite the toxicity of the material.
Zinc salts and oxides are among the compounds that contribute the most to tire toxicity [2]. Fomina et al. [32] concluded that acidolysis (protonation) is the main mechanism of zinc dissolution. It is also known that zinc oxide can be solubilized by organic acids [33]. Although toxic in high concentrations, Zinc is an essential metal for the growth of fungi [34], so it might be assimilated and used by the identified organisms.
On the other hand, proton and acid production in fungi is complex [35]. It is known that a carbon source is needed to produce these metabolites [36]. As stated above, metal mobilization can occur by acidification and ligand-promoted metal complex formation. These processes include the production of primary and secondary metabolites with chelating properties, such as organic, amino and phenolic acids [32].
The increase in growth of the morphotypes evaluated in GTR agar was maintained up to a concentration of 15% (m/v). This could be due to a balance between toxic compounds that inhibit growth and the carbon source. It is likely that by increasing the concentration of GTR to 45 (m/v) and 100% by mass, the toxic compounds limit fungal growth. Contrastingly, it is possible that with concentrations lower than 15% (m/v) the carbon source was not enough to support greater growth of the morphotypes.
The 0.6% and 1.5% (m/v) GTR media had only up to 2% of carbon source, assuming GTR as carbon and adding 0.5% (m/v) glucose. Basu et al. [37] state that, for good growth, fungi require a high carbon source; For example, potato dextrose agar (PDA), Czapek Dox agar (CzA), or oatmeal agar (OA) media contain approximately 2.4%, 3%, and 6%, respectively, of carbon source depending on the manufacturer and its formulation. These percentages do not differ from the one estimated for the concentrations of GTR agar. Nevertheless, due to the recalcitrant nature and low solubility of this polymer, the availability and access of the carbon source could be compromised.
The formation of a biofilm on the polymer surface is the first step of biodegradation [38,39] (Figure 2C). Fungi can degrade a damaged or rough surface more easily than smooth surfaces because the propagules are more likely retained on the surface [40]. Rough or cracked surfaces concentrate nutrients and moisture, providing favorable conditions for fungal attachment and growth [1]. The microorganisms that colonize the surface adhere utilizing extracellular polymeric substances, mainly polysaccharides. Fungi can form hydrophobic proteins capable of binding to the polymer surface, producing degrading enzymes, and growing rapidly and efficiently by spreading and penetrating the substrate with their mycelial network [38]. The samples of the material subjected to fungal treatment showed mycelial growth. The growth was observed through confocal microscopy using Calcofluor, a fluorochrome used for the detection of microorganisms, particularly those that have cellulose or chitin in their wall (MERCK, 2021) (Figure 5) and by MBE (Figure 6). The mycelia could be observed on the GTR samples even after the material was washed, vortexed, centrifuged, sterilized by autoclave, and dried at 45°C. Shah et al. [1], mention that some additives such as fillers and plugs can promote the biodegradation of rubber materials. Meanwhile, Lucas et al. [8] state that the development of microorganisms on the surface is an indicator of polymer biodeterioration. Consequently, all isolates evaluated have the potential to biodeteriorate tire rubber.
Before the assimilation of the GTR by fungi, it must be depolymerized. Depolymerization occurs mainly due to oxidoreductase and hydrolase enzymes [8]. Microorganisms secrete enzymes that form free radicals capable of breaking down polymeric molecules, progressively reducing their molecular weight. This process generates oligomers, dimers, and monomers that can enter the cell and then be used as an energy source [38]. The weight of the GTR as a response variable was not considered since it was not possible to separate the GTR from the biomass after incubation.
The three-dimensional structure acquired after vulcanization is one of the greatest challenges for tire biodegradation [5]. However, in nature, there are compounds with this structure, such as lignin. Ligninolytic microorganisms synthesize enzymes capable of breaking complex compounds [8]. The main enzymes involved are lignin peroxidases, manganese peroxidases and laccases [41]. Peroxidases and laccases have been used for the degradation of other recalcitrant compounds, such as polyethylene [29,42], natural rubber [17] and other polymers [43]. It is likely that these enzymes, capable of interacting with many different substrates, are also involved in the depolymerization of tire rubber.
All the genera of the selected and identified morphotypes have reported the production of at least one of these enzymes. Curvularia species produce laccases [44], cellulases and manganese peroxidases [45]. Species of the genus Mucor can produce enzymes such as amylases and even laccases [46,47]. Species of Aspergillus are considered a source of new laccases and can produce peroxidases [48,49]. Finally, lytic enzymes such as hydrolases, peroxidases and laccases are also produced in the genus Trichoderma [50,51].
Polycyclic aromatic hydrocarbons (PAHs), present in tires, are largely responsible for their toxicity [14]. Most fungi cannot use PAHs as sole carbon source but can cometabolize PAHs to a wide variety of oxidized products and, in some cases, to CO2. Besides that, it is known that ligninolytic fungi can metabolize PAHs with the help of enzymes such as laccases. Still, non-ligninolytic fungi can generally metabolize PAHs through monooxygenases and cytochrome P450 epoxide hydrolase, to form trans-dihydrodiols [43,52,53]. There are reports of these enzymes for species of Trichoderma, Curvularia, Mucor and Aspergillus [51,54-57]. There are also records of PAHs degradation [52]. The morphotypes identified in this work can grow in GTR agar, despite the presence of PAHs and other toxic compounds. It is possible that the genera identified as Trichoderma sp. and Mucor sp.1 effectively grew in GTR agar at different concentrations, due to their tolerance to these compounds. Hence, their growing capacity may be related to the ability to co-metabolize PAHs.
Most fungi generated a decrease in the pH of the medium, compared to the control. This indicates the production of organic acids and the use of the substrate. It may also signify the ability to solubilize and, possibly, assimilate toxic metals present in tires [32]. Regardless, not all morphotypes able to acidify their medium managed to colonize GTR agar at a concentration of 100% (m/v). This may happen due to a lack of the enzymatic machinery needed to depolymerize the carbon from the GTR, which would be necessary to support fungal growth. Another important consideration is that at this concentration, toxic compounds such as PAHs may be high enough to limit growth. This statement is more likely since the morphotypes showed better growth a GTR concentration of 15% (m/v).
The degradation of GTR could be verified with FTIR-ATR, a method that allows non-destructive in situ analysis of surfaces covered by microbial biofilms [39]. Bands are observed above 3400 cm-1, a signal corresponding to OH bonds [31] and a change in the signal between 600 and 1000 cm-1 corresponding to stretching of the C-O bond, which suggests oxidation of the GTR by the fungi studied. Rose et al. [58], mention the presence of aldehyde and ketone groups as products of the oxidative degradation of cis-1,4-isoprene, the main component of rubber [59,60].
Finally, several morphotypes with rubber tire degradation potential were species of Trichoderma. This genus of saprophytes, with minimal nutritional requirements and fast growth, produces a wide variety of secondary metabolites. As mentioned above, these metabolites may promote the solubilization of metals by chelating them and acidifying the medium. Transformation and even degradation of other components that, like tires, are potentially dangerous and recalcitrant have been reported for this genus, which is considered a hyperproducer of degradative enzymes.
We thank the microbiologist and microscopist Humberto Ibarra-Ávila for his collaboration in the interpretation of the images obtained under optical, confocal and scanning electron microscopy.
The authors declare that they have no conflicts of interest.
Citation: Gómez-Gómez S, Gómez-Méndez LD, Alvarado-Fernández AM (2022) Degrading Tire Rubber: Could Isolation of Filamentous Fungi Work? Fungal Genom Biol. 12:201.
Received: 17-Aug-2022, Manuscript No. FGB-22-18910; Editor assigned: 22-Aug-2022, Pre QC No. FGB-22-18910 (PQ); Reviewed: 05-Sep-2022, QC No. FGB-22-18910; Revised: 12-Sep-2022, Manuscript No. FGB-22-18910 (R); Published: 19-Sep-2022 , DOI: 10.35841/2165-8056.22.12.201
Copyright: © 2022 Gómez-Gómez S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.