Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2020)Volume 11, Issue 2
Parasitological examination of Paramormyrops kingsleyae Günther, 1896 caught in the Djolon River in Cameroon revealed the presence of three new species of Myxosporidia of the genus Myxidium Bütschli 1882 and Henneguya Thelohan, 1892 of which complete description is given in the present study. These species are: Myxidium binguelai sp. nov., parasite of the kidney that produce fusiform spores with a turgid medial part and both ends pointed. They measured 12.1 (11.1-12.9) μm long x 5.3 (4.8-5.8) μm broad; the polar capsules are ovoid, equal in size and measure 3.6 (3.0-4.1) μm x 2.5 (2.0-2.8) μm. Myxidium djolonensis sp. nov., a parasite of the gall bladder and urinary bladder, produce fusiform and elongated spores, that measure 16.3 (15.5-18.0) μm long x 6.1 (5-7.1) μm broad; its polar capsules are equal and measure 4.9 (4-5.6) x 3.5 (3-4.2) μm. Henneguya paramormyropsi sp. nov. form cyst in gill, eye and kidney. The total spore length is 29.2 (25.5-32.5) μm. The ovoid spore body, with narrowed anterior end measure 11.9 (10.7-12.8) μm x 4.5 (3.9-5.9) μm. The two separate caudal appendages measure 17.3 (14-20.3) μm. The 2 equal-sized thin and smooth valves surrounded 2 equal-sized pyriform, elongate polar capsules (5 (4.2-5.6) ×1.5 (1.2-1.9) μm) that contained 3 to 4 coils of the filament.
Myxozoa; Myxidium binguelai sp. nov.; Myxidium djolonensis sp. nov.; Henneguya paramormyropsi sp. nov.; parasite; Paramormyrops kingsleyae; Cameroon
Fish farming is one of the most important human activities because of the growing need of animal protein in the world [1]. Despite its food and economic importance, fish is also a favorable biotope for the development of a large number of parasites among which Myxosporidia [2,3].
Discovered by Müller [4], Myxosporidia Bütschli [5] are ubiquitous metazoans parasites of Vertebrates and Invertebrates [2]. They are of great importance in ichthyopathology because of lethal and sublethal effects related to their development in host fish, especially in fish farming [6]. More than a century after their first description based on the morphological and metrical characteristics of the spore, the worldwide Myxosporidia fauna is now estimated at 2200 species [7]. They represent 18% of Cnidaria species currently described and are distributed in 64 genera and 17 families [7]. Myxosporidia fauna in African freshwater fishes is estimated at about 280 species [8-12], while that of freshwater fish from Cameroon is represented by about 80 species of which 11 belong to the genus Myxidium [11] and 12 to the genus Henneguya [8].
Molnár [13] believes that, most Myxosporidia species have strict specificity towards their hosts. In addition, Ali et al. [14] suggest the existence of geographical barriers between species of Myxosporidia. Thus, taking into consideration the fish species richness, the diversity of the environment and the geographical extent of the continent, the Myxosporidia biodiversity in Africa is certainly underestimated. Several Myxosporidia species have been described on Mormyrid fish in African [15]. However, no data are available on these parasites on Paramormyrops kingsleyae Günther [16] (Mormyridae).
In a more general study of myxosporidia parasites of some food and economic importance Teleosts in Cameroon, we found for the first time in P. kingsleyae, three new species whose full description is given in the present work. These species are: Myxidium binguelai sp. nov., Myxidium djolonensis sp. nov. and Henneguya paramormyropsi sp. nov.
Fish examined during this study were harvested from March to July 2018 in the Djolon River (tributary of the Nyong River) at Binguela, a village in the Mbankomo District in the Mefou and Akono Division, Central Region of Cameroon. This village is located between 3°31' to 4° North latitude and 11°13' to 12° East longitude.
Fish catches were done using gill nets. In the field, once fish were caught, a buttonhole was made on the ventral surface of each individual. They were immediately immersed in a 10% formalin solution before being brought to the laboratory. Systematic position of sampled fish was taken from Stiassny et al. [17]. In the laboratory, the fish were firstly examined with naked eye (eyes, fins, operculum, scales, skin) and then with the Olympus BO61 binocular stereoscopic microscope for the presence of cysts. After dissection of the fish hosts, internal organs such as gills, heart, liver, kidneys, spleen, gall bladder, urinary bladder, gonads, intestine and urethra were taken individually and examined. The contents of the cysts were identified with the objective 100x of an IVYMEN light microscope. The content of the gall bladder, urinary bladder and swim bladder was also examined between glass and glass cover slide under the microscope. Smears from kidney, spleen, liver, gonads, heart and urethra were carefully examined at the 40x objective of the microscope. Spore smears were fixed with methanol and stained with May-Grünwald-Giemsa. Drawings of unstained spores were performed using a Wild M-20 microscope equipped with a camera Lucida. Fifty spores were measured using an objective micrometer. Variables taken into consideration were those proposed by Lom et al. [18]. Microphotographs of spores were performed using an Olympus BH-2 microscope equipped with a microphotograph device.
Myxidium binguelai sp. nov.m
Vegetative form: Not observed; numerous spores are dispersed in the kidney.
Spore: Small in size (12.1 x 5.3 μm on average), spores are fusiform with a turgid medial part and both ends pointed (Figures 1 and 2). Each valve carries 6 to 7 longitudinal striations (Figures 2 and 3). The suture line is straight. Polar capsules are ovoid, of equal size and open at the opposite extremities of the spore (Figures 4 and 5). Within each of them, there are 4 coils of the filament (Figure 5). The sporoplasm, located in the extra-capsular space, is finely granular and contains a large iodinophilous vacuole (Figures 1, 2 and 5).
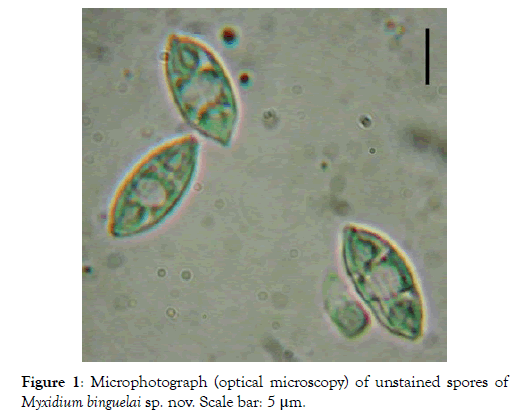
Figure 1: Microphotograph (optical microscopy) of unstained spores of Myxidium binguelai sp. nov. Scale bar: 5 μm.
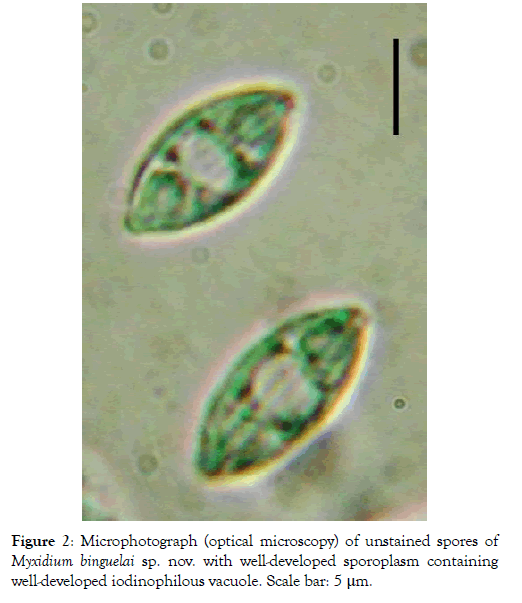
Figure 2: Microphotograph (optical microscopy) of unstained spores of Myxidium binguelai sp. nov. with well-developed sporoplasm containing well-developed iodinophilous vacuole. Scale bar: 5 μm.
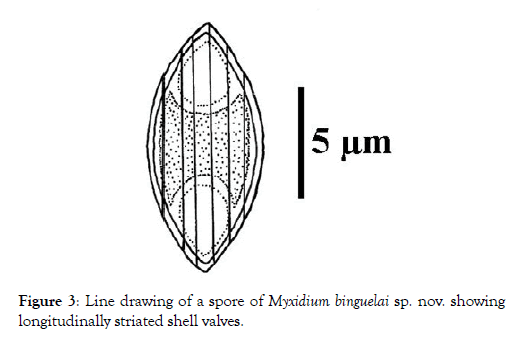
Figure 3: Line drawing of a spore of Myxidium binguelai sp. nov. showing longitudinally striated shell valves.
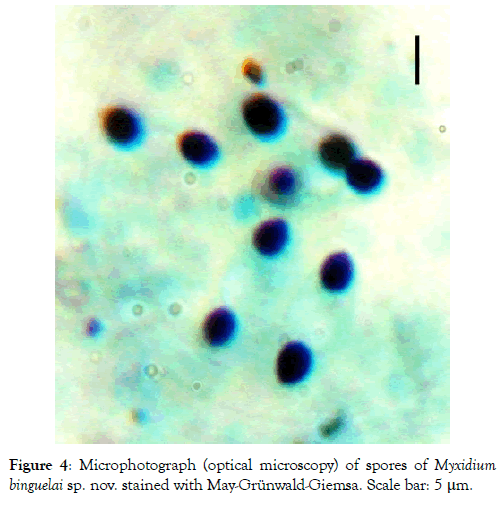
Figure 4: Microphotograph (optical microscopy) of spores of Myxidium binguelai sp. nov. stained with May-Grünwald-Giemsa. Scale bar: 5 μm.
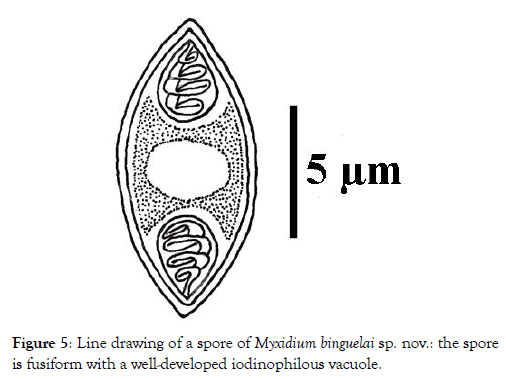
Figure 5: Line drawing of a spore of Myxidium binguelai sp. nov.: the spore is fusiform with a well-developed iodinophilous vacuole.
Measurements: Measurements are given in Table 1.
Table 1: Comparative description of Myxidium binguelai sp. nov. with morphologically similar species (measurements in micrometre).
| Myxidium species | Host | Site of infection | Country | LS | WS | LPC | WPC | FC | StV | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| M. binguelai sp. nov. | Paramormyrops kingsleyae | kidneys | Cameroon | 12.1 (11.1-12.9) | 5.3 (4.8-5.8) | 3.6 (3.0-4.1) | 2.5 (2.0-2.8) | 4 | 6-7 | Present study |
| M. clariae | Clarias lazera | Gall bladder | Israel | 13.4–15.1 | 4.5–6.0 | 3.6–4.8 | 3.0–3.8 | 5-6 | 7-9 | Landsberg [25] |
| M. distichodi | Distichodus engycephalus | Gall bladder | Chad | 16.3 (16-17) | 6.5 (6-7) | 4.9 (4.5-5.5) | 3.2 (3-3.5) | - | ± | Kostoïngué et al. [24] |
| M. chrysichthyi | Chrysichthys nigrodigitatus | Gall bladder | Benin | 14.4 (13-16) | 8.3 (7-10) | 3.6 (2.5-4.5) | 2.3 (2-2.5) | - | - | Sakiti [23] |
| M. mendehi | Barbus guirali, B. martorelli | Kidneys | Cameroon | 9.9 (7.8-13.2) | 4.1 (3.1-4.9) | 3.4 (2.7-4.5) | 2.3 (1.8 –3.1) | 5-7 | 5 | Fomena et al. [19] |
| M. nkamensis | Clarias pachynema | Gall bladder | Cameroon | 25.4 (24-27.5) | 11.7(10-13) | 10.2 (10-11) | 7.7 (7-8) | 10-12 | 10-13 | Fomena et al. [21] |
| M. schalli | Synodontis shall | Gall bladder | Egypt | 11-12.5 | 4.5-6.5 | 2.5-3.5 | 1.5-2 | - | - | Ghaffar et al. [22] |
| Myxidium sp. | Tilapia zillii | Liver ; kidney | Burkina Faso | 12 (11-13) | 4.8 (4-6) | 2.9 (2.5-3.5) | 1.9 (1.5-2) | 4-5 | ± | Kabré [20] |
Averages of the parameters measured are followed by minimal and maximal values in brackets.
LS: length of the spore; WS: width of the spore; LPC: length of the polar capsules; WPC: width of the polar capsules; FC: number of polar filament coils; StV: Number of striations on shell valves; –: absent or not reported in the species description; ±: shell valves striations are present, but the number was not reported in the species description.
Type host: Paramormyrops kingsleyae Günther [16] (Mormyridae).
Type locality: Binguela (3° 42' 11” N; 11° 22' 34” E) in Djolon River (Center Region; Cameroon).
Location in the host: kidney.
Prevalence: 18.8% (13 parasitized fish out of 69 examined).
Type material: Glass slides with stained spores (syntype) and parasitized kidney preserve in 10% buffered neutral formalin are deposited in the parasitological collection of the Laboratory of Parasitology and Ecology, Faculty of Science, University of Yaounde 1, Cameroon (No. Myxo/2019/LPE-001).
Etymology: The species is related to the locality of Binguela where host fish were captured.
Myxidium djolonensis sp. nov.
Vegetative form: Not observed, only mature and free spores were found in the bile and urinary bladder.
Spores: Fusiform, elongated (2.7 times longer than wide) and sometimes slightly arched, the spores have truncated ends (Figures 6-8). Each valve has 7 to 9 longitudinal striations (Figures 7 and 8). The polar capsules are ovoid and equal in size (Figures 9 and 10). Within each of them, there are 5 to 7 coils of the filament (Figure 10). The sporoplasm occupies the extracapsular space.
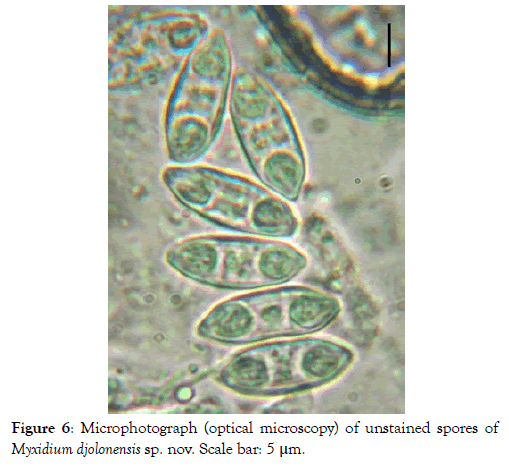
Figure 6: Microphotograph (optical microscopy) of unstained spores of Myxidium djolonensis sp. nov. Scale bar: 5 μm.

Figure 7: Microphotograph (optical microscopy) of unstained spore of Myxidium djolonensis sp. nov. : spore with truncated ends. Scale bar: 5 μm.
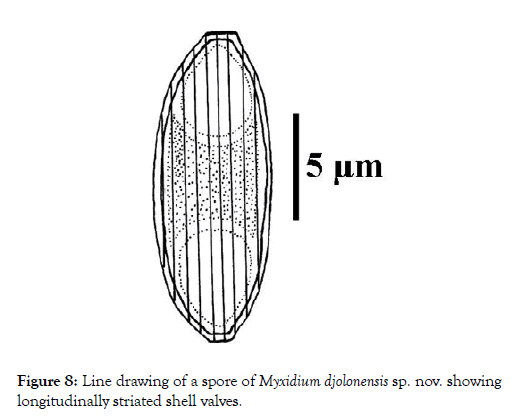
Figure 8: Line drawing of a spore of Myxidium djolonensis sp. nov. showing longitudinally striated shell valves.
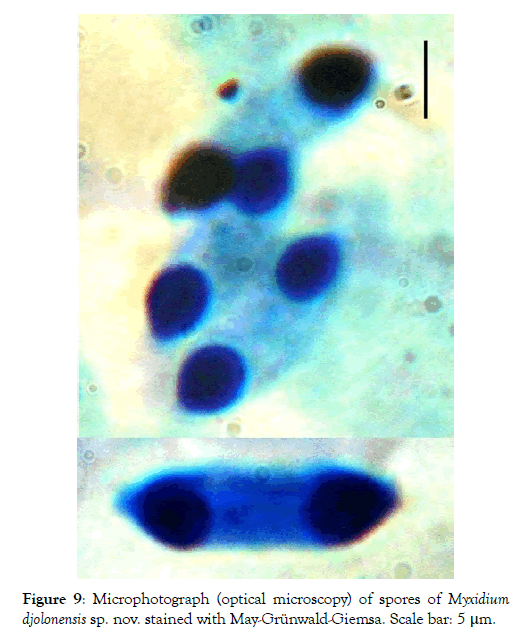
Figure 9: Microphotograph (optical microscopy) of spores of Myxidium djolonensis sp. nov. stained with May-Grünwald-Giemsa. Scale bar: 5 μm.
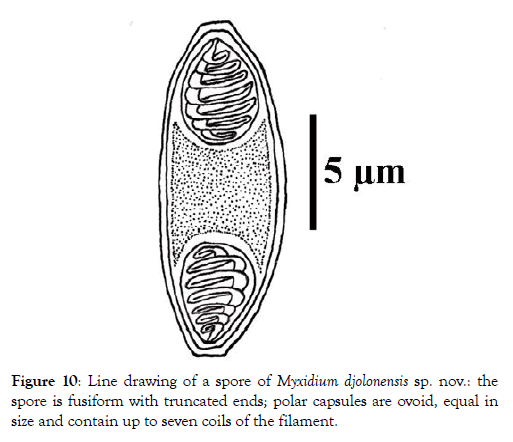
Figure 10: Line drawing of a spore of My xidium djolonensis sp. nov.: the spore is fusiform with truncated ends; polar capsules are ovoid, equal in size and contain up to seven coils of the filament.
Measurements: Measurements are given in Table 2.
Table 2: Comparative description of Myxidium djolonensis sp. nov. with morphologically similar species (measurements in micrometre).
| Myxidium species | Host | Site of infection | Country | LS | WS | LPC | WPC | FC | StV | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| M. djolonensis sp. nov. | Paramormyrops kingsleyae | Gall bladder; urinary bladder | Cameroon | 16.3 (15.5-18.0) | 6.1 (5-7.1) | 4.9 (4-5.6) | 3.5 (3-4.2) | 5-7 | 7-9 | Present study |
| M. brienomyri | Brienomyrus brachyistus | Gall bladder | Cameroon | 13.7 (12.2-16.2) | 6.5 (5.5-9) | 4.2 (3.5-5) | 4.2 (3.5-5) | 3-5 | 6-12 | Fomena et al.[27] |
| M. clariae | Clarias lazera | Gall bladder | Israel | 13.4–15.1 | 4.5–6.0 | 3.6–4.8 | 3.0–3.8 | 5-6 | 7-9 | Landsberg [25] |
| M. distichodi | Distichodus engycephalus | Gall bladder | Chad | 16.3 (16-17) | 6.5 (6-7) | 4.9 (4.5-5.5) | 3.2 (3-3.5) | - | ± | Kostoïngue et al. [24] |
| M. molnari | Cirrhina reba | Gall bladder | India | 10.9 (8.3–10.0) | 3.8 (2.9–5.0) | 3.9 (3.3–5.0) | 2.5 (2.0–3.3) | 6-7 | Yatindra et al. [26] | |
| M. nkamensis | Clarias pachynema | Gall bladder | Cameroon | 25.4 (24-27.5) | 11.7(10-13) | 10.2 (10-11) | 7.7 (7-8) | 10-12 | 10-13 | Fomena et al. [21] |
| M. sangei | Parachanna obscura | Gall bladder | Cameroon | 13.3 (12-14.5) | 4.2 (3.5-5) | 4.3 (4-5) | 2.9 (2.8-3) | 3-4 | 5-6 | Fomena et al. [21] |
Averages of the parameters measured are followed by minimal and maximal values in brackets.
LS: length of the spore; WS: width of the spore; LPC: length of the polar capsules; WPC: width of the polar capsules; FC: number of polar filament coils; StV: Number of striations on shell valves; –: absent or not reported in the species description; ±: shell valves striations are present, but the number was not reported in the species description.
Type host: Paramormyrops kingsleyae Günther [16] (Mormyridae).
Type locality: Binguela (3° 42' 11” N; 11° 22' 34” E) in Djolon River (Center Region; Cameroon).
Locations in the host: Gall bladder and urinary bladder.
Prevalence: 24.6% (17 parasitized fish out of 69 examined).
Type material: Glass slides with stained spores (syntype) and parasitized organs (gall bladder and urinary bladder) preserve in 10% buffered neutral formalin are deposited in the parasitological collection of the Laboratory of Parasitology and Ecology, Faculty of Science, University of Yaounde 1, Cameroon (No. Myxo/2019/ LPE-002).
Etymology: The species is related to the Djolon River in which host fish were captured.
Henneguya paramormyropsi sp. nov.
Vegetative form: Elongated, spherical or subspherical whitish cysts were observed in the secondary gill lamellae and the aqueous humor of the eye. These cysts are polysporous and measure 590-396 x 310-252 μm. On a parasitized host fish, one can count an average of 10 cysts. Vegetative stages were not observed in the kidney but isolated or grouped spores were found.
Spore: Spore body ovoid with narrowed anterior end (Figure 11). Shell valves smooth and extend posteriorly with two separate caudal appendages (Figure 11). Polar capsules are pyriform, elongated (3.3 times longer than wide) and of equal size (Figures 12 and 13). In each of them, there are three to four coils of the filament (Figure 14). The sporoplasm occupies the rest of the spore cavity (Figures 11 and 14).
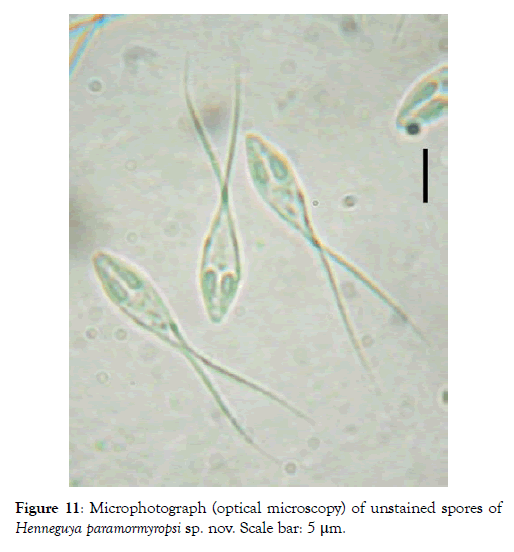
Figure 11: Microphotograph (optical microscopy) of unstained spores of Henneguya paramormyropsi sp. nov. Scale bar: 5 μm.
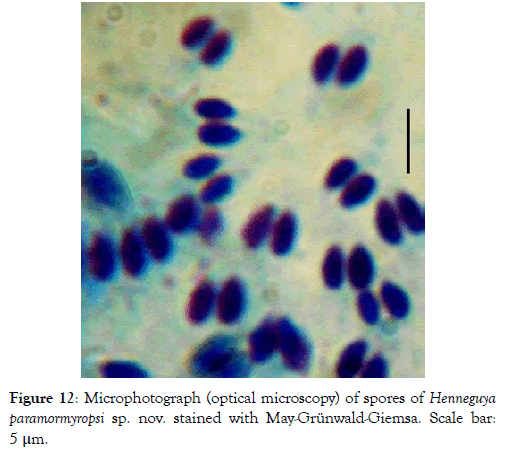
Figure 12: Microphotograph (optical microscopy) of spores of Henneguya paramormyropsi sp. nov. stained with May-Grünwald-Giemsa. Scale bar: 5 μm.
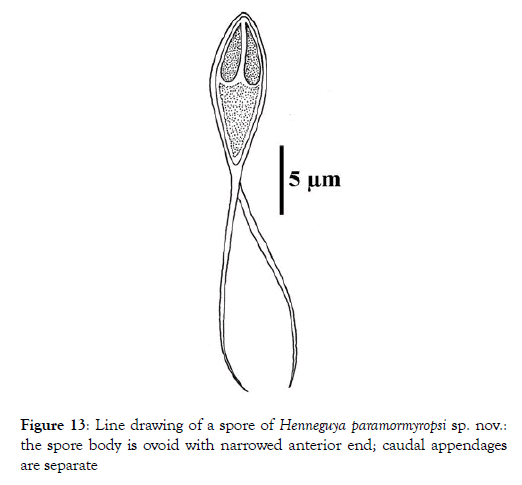
Figure 13: Line drawing of a spore of Henneguya paramormyropsi sp. nov.: the spore body is ovoid with narrowed anterior end; caudal appendages are separate
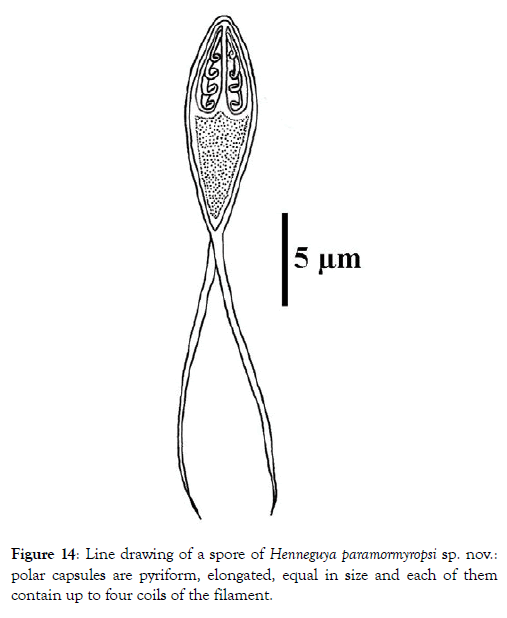
Figure 14: Line drawing of a spore of Henneguya paramormyropsi sp. nov.: polar capsules are pyriform, elongated, equal in size and each of them contain up to four coils of the filament.
Measurements: Measurements are given in Table 3.
Table 3: Comparative description of Henneguya paramormyropsi sp. nov. with morphologically similar species (measurements in micrometre).
| Henneguya species | Host | Site of infection | Country | LSB | WSB | PC | LPC | WPC | LS | LCA | FC | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H. paramormyropsi sp. nov. | Paramormyrops kingsleyae | Gills, eye, kidney | Cameroon | 11.9 (10.7-12.8) | 4.5 (3.9-5.9) | = | 5 (4.2-5.6) | 1.5 (1.2-1.9) | 29.2 (25.5-32.5) | 17.3 (14-20.3) | 3-4 | Present study |
| H. dini | Heterotis niloticus | Gills | Burkina-Faso | 11.47 (11-12) | 3.47 (3-5) | = | 3.43 (3-4) | 1.02 (1-1.5) | 29.73 (27-32) | 18.37 (16-20) | 5-8 | Kabré et al. [31] |
| H. mailaoensis | Mormyrus cashive | Gills | Chad | 17.6 (15-18) | 5.7 (5-6) | = | 5.9 (5-7) | 2.2 (2-3) | 61.7 (58-63) |
44.5 (41-46) | - | Kostoïngué et al. [29] |
| H. mormyri | Mormyrus cashive | Gills | Chad | 8.4 (8-9) | 4.5 (4-5) | = | 3.3 (3-4) | 1.9 (1-3) | 32.2 (30-34) | 23.1 (23-25) | 3-4 | Kostoïngué et al. [29] |
| H. ntondei | Schilbe mystus | Gills | Cameroon | 12.8 (10.5-14) | 5.4 (4-7) | = | 6.4 (5.5-8) | 1.7 (1.2-2) | 27.7 (24-35) | 14.8 (10-23) | 10 | Fomena et al. [30] |
| H. nyongensis | Marcusenius moorii | Gills; muscles | Cameroon | 12.6 (10-14) | 5.4 (4.5-6.5) | = | 6.2 (5.5-7) | 2.3 (2-2.8) | 33.6 (30.5-36.5) | 21 (20-23.5) | 4-5 | Fomena et al. [28] |
| H. odzai | Marcusenius moorii | Gills | Cameroon | 14.4 (13-16) | 3.9 (3.3-4.6) | = | 3.9 (3.2-4.9) | 1.3 (1-1.6) | 32.9 (29.2-36) | 18.5 (15-21.5) | 4 | Fomena et al. [28] |
| H. suprabranchiae | Clarias lazera | Accessory breathing organ | Israel | 13.5 (12.2-14.3) | 6.4 (5.6-6.9) | ≠ | 7.6 (7.0-8.1)* 7.3 (6.6-8.0)** | 2.1 (1.8-2.3) | 37.5 (30.7-43.3) | 24.0 (18.5- 29.0) | - | Landsberg [25] |
Averages of the parameters measured are followed by minimal and maximal values in brackets.
LSB: length of the spore body; WSB: width of the spore body; PC: relative length of the polar capsules (=: equal; ≠ unequal); LPC: length of the polar capsules; WPC: width of the polar capsules; LS: total length of the spore; LCA: length of the caudal appendages; FC: number of polar filament coils (-: not reported in the species description); * Referring to the largest polar capsule; ** referring to the smallest polar capsules.
Type host: Paramormyrops kingsleyae Günther [16] (Mormyridae).
Type locality: Binguela (3° 42' 11” N; 11° 22' 34” E) in Djolon River (Center Region; Cameroon).
Locations in the host: Gills, eye and kidney.
Prevalence: 27.5 % (19 parasitized fish out of 69 examined).
Type material: Glass slides with stained spores (syntype) and cyst preserve in 10% buffered neutral formalin are deposited in the parasitological collection of the Laboratory of Parasitology and Ecology, Faculty of Science, University of Yaounde 1, Cameroon (No. Myxo/2019/LPE-003).
Etymology: The species is related to the generic name of the host fish.
Myxidium binguelai sp. nov.
In Africa, two Myxosporidia species belonging to the genus Myxidium have been described in the kidney of freshwater fishes, these are: Myxidium mendehei Fomena and Bouix [19], parasite of Enteromius guirali and E. martorelli in Cameroon; Myxidium sp. Kabré [20], parasite of the kidney and liver of Tilapia zillii in Burkina-Faso. The spores of M. mendehei are significantly smaller [9.9 (7.8-13.2) x 4.1 (3.1-4.9) μm] than those presently described. The general shape of the spore of Myxidium sp. recalls that of the species we are describing; however, this parasite forms arched spores with narrowed medial part and narrower polar capsules (1.5-2 μm).
Regarding the general shape of the spore, our parasite is close to Myxidium nkamensis Fomena, Lekeufack Folefack and Bouix [21] (host: Clarias pachynema (Clariidae) in Cameroon), M. schalli Ghaffar, El-Shahawi and Naas [22] (host: Synodontis schall (Mochokidae) in Egypt) and Myxidium chrysichthyi Sakiti [23] (hosts: Chrysichthys nigrodigitatus and C. auratus (Bagridae) in Benin). Myxidium nkamensis forms significantly larger spores that are 25.4 (24 -27.5) μm long and 10.2 (10-11) μm wide. Its polar capsules are more developed (10.2 x 7.7 μm on average). Myxidium schalli forms spores of comparable size (12.3 x 5.4 μm on average) to those of the parasite described here. However, its polar capsules are pear-shaped. No data were provided on the number of valvular striations and the number of loops of the filament within the polar capsules when describing M. schalli. The spores of M. chrysichthyi are more developed (14.4 x 8.39 μm on average).
Although spindle-shaped with pointed ends, our spores, are less developed compared to those of Myxidium distichodi Kostoïngué, Faye and Toguebaye [24] (host: Distichodus engycephalus in Chad) which measure 16.34 × 6.53 μm on average. The spores of Myxidium clariae Landsberg [25] (host: Clarias lazera (synonym: Clarias gariepinus) in Israel) are similar in shape to those of the species presently described, but they are longer (14, 3 (13, 4 -15, 1) μm) and their polar capsules are more developed (4.2 (3.6-4.8) x 3.3 (3-3.8) μm).
The parasite of Paramormyrops kingsleyae differs from species of Myxosporidia previously described by many characters. We believe that, this parasite is new and propose to name it Myxidium binguelai sp. nov., a name that refers to the locality of Binguela where fish hosts were captured.
Myxidium djolonensis sp. nov.
With the general shape of its spore, the presently described Myxosporidia approaches species previously described in Africa or other continents. Thus, the spores of Myxidium nkamensis Fomena, Lekeufack Folefack and Bouix [21] (host: Clarias pachynema (Clariidae) in Cameroon), Myxidium molnari Yatindra and Mathur [26] (host: Cirrhina reba (Cyprinidae) in India) recall by their shape, that of the species here described. However, the spores of M. nkamensis are more developed (25.4 x 11.7 μm on average). Myxidium molnari produces less developed spores (10.42 x 3.81 μm on average) with polar capsules measuring 3.93 x 2.5 μm on average.
Because of their shape sometimes arched, the spores of the presently described species recall those of Myxidium clariae Landsberg [25] and Myxidium sangei Fomena, Lekeufack Folefack and Bouix [21]. However, M. clariae, described in the gallbladder of Clarias lazera (synonym: Clarias gariepinus) in Israel forms less developed spores (14.3 x 5.3 μm on average), with pointed extremities and up to 9 striations per valve. M. sangei, described on Parachanna obscura (Chanidae) in Cameroon, forms significantly smaller spores that are 13.3 (12-14.5) μm long and 4.2 (3.5-5) μm wide.
M. distichodi Kostoïngué, Faye and Toguebaye [24] (host: Distichodus engyephalus (Cyprinidae) in Chad) forms spores whose length (16.3 x 6.5 μm on average) is comparable to that of the spores of the species presently describe. However, the spores of M. distichodi are spindle-shaped, with pointed ends and a turgid medial part.
Myxidium brienomyri forms large trophozoites in the gall bladder of Brienomyrus brachyistus (Mormyridae) in Cameroon [27]. The spores of this species, being ellipsoid with rounded ends measured 13.7 x 6.47 μm on average. The maximum number of striations found on the valves is greater (12 striations) compared to 9 striations found on spores of the presently described species. In addition, their polar capsules are spherical.
These differences between the parasite of Paramormyrops kingsleyae and previously described species lead to the creation of a new species. We propose to name it Myxidium djolonensis sp. nov., a name that refers to the Djolon River in which host fish were caught.
Henneguya paramormyropsi sp. nov.
Four species of Henneguya were previously described in Mormyrid fish in Africa. Henneguya nyongensis Fomena and Bouix [28] forms innumerable cysts between secondary gill lamellae of Marcusenius moorii (Mormyridae) in Cameroon. The spores of this species are longer (33 μm on average), with longer caudal appendages (20 to 23.5 μm). The polar capsules are more developed (6.2 × 2.3 μm on average) and open to the anterior end by a "neck like" structure. Henneguya odzai Fomena and Bouix [28] parasitizes the gills of Marcusenius moorii (Mormyridae) in Cameroon. Although having a total length of 29.2 to 36 μm, the spores of H. odzai have elongated spore body (3.69 times longer than wide) and a broad and rounded anterior end. Kostoïngué et al. [29] described Henneguya mormyri and Henneguya mailaoensis on the gills of Mormyrus cashive (Mormyridae) in Chad. With comparable general spore morphology, H. mormyri have a less developed spore body (8-9 x 4-5 μm) and shorter polar capsules (3-4 μm). The spores of Henneguya mailaoensis are longer (58 to 63 μm) with a more developed spore body (17.6 μm long).
The general morphology of our spores recalls that of Henneguya ntondei Fomena, Lekeufack Folefack and Bouix [30], a parasite of the gills of Schilbe mystus (Schilbeidae) in Cameroon. However, the spore of H. ntondei has shorter caudal extensions (14.8 μm on average) and longer polar capsules (6.4 μm on average) containing 10 coils of the filament.
Henneguya suprabranchiae Landsberg [25], parasitizes tree organs of Clarias lazera in Israel. Although of comparable general morphology, the spore of H. suprabranchiae has a more developed spore body (13.5 x 6.4 μm on average) and longer polar capsules (7-8.1 μm) containing 9 to 10 coils of the filament.
Henneguya dini Kabre et al. [31], a gill parasite of Heterotis niloticus (Osteoglossidae) in Burkina-Faso, differs from our parasite by its spore body which is 3.3 times longer than wide and less wide (3.43 μm on average). Moreover, their polar capsules are less developed (3.43 × 1.02 μm on average).
The parasite of Paramormyrops kingsleyae, which differs from known Myxosporidia species, is new. We propose to name it Henneguya paramormyropsi sp. nov. with respect to the generic name of the host fish.
In ichthyopathology, it is important to report new parasites and pathological conditions when they are discovered because such information may be useful in the future as a baseline for assessing the health of ecosystems in the face of global warming. Thus, the present work makes available new data on Myxosporidia parasites of Paramormyrops kinsleyae. Myxidium binguelai sp. nov., a parasite of the kidney of P. kinsleyae contains a large iodinophilous vacuole in its sporoplasm. Although the iodinophilous vacuole is not a species identification criterion for Myxobolidae, it is important to note that this is the first time that the iodinophilous vacuole is reported in a species of the genus Myxidium. The description of Myxidium binguelai sp. nov. and Myxidium djolonensis sp. nov. bring to 20 the number of species of Myxidium known in African freshwater fish and to 13 the number of species of the genus described in freshwater teleosts of Cameroon. With the description of Henneguya paramormyropsi sp. nov., 28 Henneguya species are known to infest freshwater fish in Africa while 13 are parasites of freshwater fish in Cameroon. In case of heavy infection, the rupture of cysts of Henneguya paramormyropsi sp. nov. implanted in the secondary gill lamellae and the aqueous humor of the eye can lead to a malfunction of these organs and may create lesions that would be gateways for secondary pathogens (Bacteria and Fungi). In view of the above-mentioned pathogenic effects, particular attention should be paid to the epidemiology of these parasite species in order to prevent their adverse effects on the population of Paramormyrops kinsleyae in the Nyong basin so as to handle this fish species in cultivation and promote fish yields.
Animals used followed a protocol approved and authorized by Institutional Animal Care and Use Committee at Animals Biology and Physiology Department, Faculty of Science, University of Yaounde I, Cameroon.
The authors are thankful to the Faculty of Science, University of Yaounde I, Yaounde-Cameroon, for providing all the facilities to complete this work.
The authors declared: There is no conflict of interest.t.
Citation: Benoît LFG, Vanessa FTC, Abraham F (2020) Description of three new species of Myxosporidia (Cnidaria: Myxobolidae) parasites of Paramormyrops kingsleyae Günther, 1896 (Mormyridae) in the Nyong basin in Cameroon. Fish Aqua J. 11:274. doi: 10.35248/2150-3508.209.11.275
Received: 18-Dec-2019 Accepted: 15-Apr-2020 Published: 22-Apr-2020 , DOI: 10.35248/2150-3508.209.11.275
Copyright: © 2019 Benoît LFG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.