Journal of Theoretical & Computational Science
Open Access
ISSN: 2376-130X
ISSN: 2376-130X
Research Article - (2024)Volume 10, Issue 3
The indazole scaffold has gained prominence in medicinal chemistry due to its diverse biological activities and potential as a pharmaceutical agent. This study delves into the design, synthesis and comprehensive evaluation of indazole derivatives, aiming to develop effective anticancer, antioxidant and antimicrobial agents. A series of computational studies, including molecular docking and Quantitative Structure–Activity Relationship (QSAR) analysis, guided the design of potent Tyrosine Threonine Kinase (TTK) inhibitors. The 2D-QSAR model (r²=0.9512) and 3D-QSAR model (q²=0.9132) demonstrated robust predictive accuracy, highlighting key structural attributes influencing biological activity. Synthetic schemes yielded three distinct series of indazole derivatives, which were subjected to bioassays for their anticancer, antioxidant and antimicrobial properties. Compounds 5b and 5'j exhibited noteworthy anticancer activity against A549 and MCF7 cell lines, surpassing certain existing drugs. Molecular dynamics simulations validated ligand stability, while Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) profiling confirmed favorable pharmacokinetic attributes. Structure-Activity Relationship (SAR) analyses revealed that specific substitutions significantly enhanced biological efficacy. These findings underscore the therapeutic potential of indazole derivatives, prepare for further in vivo and clinical investigations.
Indazole; Anticancer agents; Antioxidant activity; Antimicrobial activity; Quantitative Structure–Activity Relationship (QSAR); Molecular docking; Molecular simulation; Tyrosine Threonine Kinase inhibitors; Absorption, Distribution, Metabolism, Excretion and Toxicity (ADMET) profiling
The indazole scaffold has garnered considerable attention in medicinal chemistry due to its diverse biological activities. The potential of indazole derivatives as assuring candidates for drug development has been underscored by a multitude of studies. Indazole, a member of the azole family, exhibited unique structural features including aromaticity and a nitrogen-containing heterocyclic ring. With ten π-electrons arranged in an aromatic heterocyclic configuration akin to pyrazole, its molecular shape and electrostatic transmission play important roles in enzyme and receptor detection and interaction. Indazoles are renowned for their diverse biological and chemical functions, offering a wide array of therapeutic applications, making them impressive candidates for pharmaceutical research and development.
In the domain of drug design, computational studies have played an important role in predicting the potential biological activities of novel compounds. These approaches enable researchers to optimize molecular structures to enhance biological efficacy, promoting a rational and efficient design process. Building upon the insights gathered from the literature review, our current research endeavors to conduct a computational study aimed at facilitating the design of encouraging candidates featuring the indazole scaffold. The study surrounds diverse synthetic strategies, including cyclization reactions condensation reactions and substitution reactions. Subsequently, the biological evaluation of these indazole derivatives becomes crucial for assessing their efficacy and safety [1].
Molecular docking and virtual screening techniques have been employed to design indazole derivatives with specific interactions with target biomolecules, thus aiding in the identification of promising candidates for further synthesis. To accomplish study, we employed bioassays and cell-based studies as standard methodologies to investigate the interactions of these compounds with specific biological targets.
Computational study
This study focused on investigating the anticancer efficacy of indazole derivatives, particularly as TTK inhibitors. The research involved the analysis of a substantial dataset comprising 109 indazole derivatives using 2D and 3D QSAR techniques. The 2D-QSAR model, constructed through Multiple Linear Regression (MLR), exhibited a high correlation coefficient (r²) of 0.9512, indicating a robust predictive accuracy. Internal (q²) and external (pred_r²) cross-validation regression coefficients of 0.8998 and 0.8661, respectively, reinforced the model's reliability. The study highlighted essential structural features for optimizing indazole as a potential anticancer agent, offering valuable insights for medicinal chemists.
In addition to the 2D-QSAR analysis, a 3D-QSAR model was developed using the SWF kNN approach, revealing a high internal cross-validation regression coefficient (q²) of 0.9132. Steric descriptors emerged as crucial factors governing the anticancer activity of the indazole derivatives, providing significant structural insights. The study emphasized the role of physicochemical and alignment-independent descriptors in influencing the 2D-QSAR model's predictive power. The research, with its meticulous approach and reliable models, contributes to the rational design of indazole compounds with enhanced TTK inhibitory activity [2].
2D and 3D QSAR studies has proven invaluable in guiding the design of synthetic schemes for Series I, Series II and Series III. By dissecting molecular structures and their corresponding activity profiles, this analysis provided important insights into the relationship between chemical features and biological activity. This strategic approach not only streamlined the synthetic process but also facilitated the creation of compounds with improved efficacy and selectivity, ultimately advancing our quest for novel therapeutic agents.
Furthermore, to evaluate their potential as pharmaceutical candidates, a comprehensive ADMET profile analysis was performed, encompassing critical attributes including ADMET effects. This assessment was carried out using the PreADMET online server. Precise physicochemical parameters were ascertained by employing the Molinspiration online methodology in conjunction with Lipinski's Rule of Five.
Synthetic schemes for series I and series II
This all-encompassing investigation involved the conception, synthesis and multifaceted assessment of an initial newer series of N-(1-(2-chloropyrimidin-4-yl)-1H-indazol-5-yl) benzenesulfonamide derivatives (4a-i) and N-(1-(2-chloropyrimidin-4-yl)-6-ethoxy-1H-indazol-5-yl) benzenesulfonamide derivatives (5a-i). A second newer series of N-(1-(2-(phenylamino) pyrimidin-4-yl)-1H-indazol-5-yl) benzenesulfonamide (6a-6n) and N-(6-ethoxy-1-(2-(phenylamino) pyrimidin-4-yl)-1H-indazol-5-yl) benzenesulfonamide (7a-7c) derivatives was also synthesized and characterized (Figure 1). These compounds were then subjected to rigorous biological evaluations, including anticancer, antioxidant and antimicrobial assays.
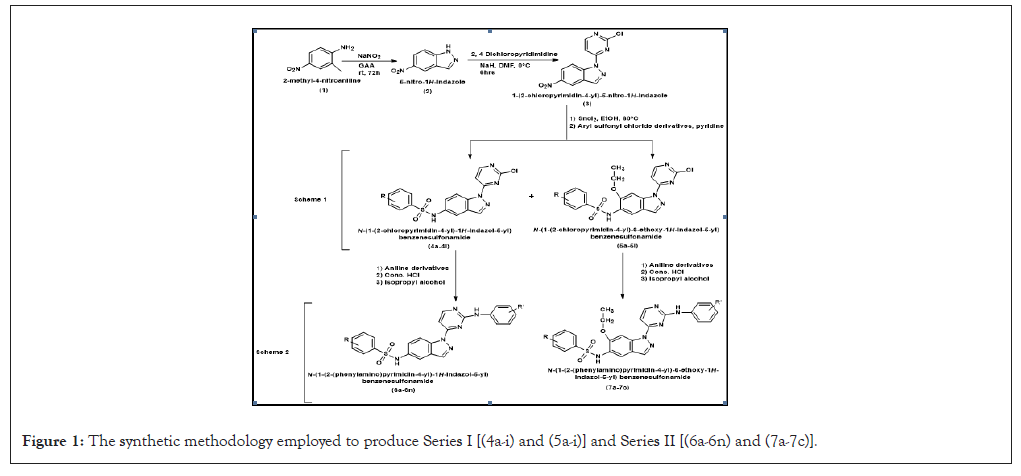
Figure 1: The synthetic methodology employed to produce Series I [(4a-i) and (5a-i)] and Series II [(6a-6n) and (7a-7c)].
From series I compound 5b, featuring a nitro group at the 2-position and an ethoxy group at the 6-position of the indazole ring and compound 5d having a methoxy group at 4-postion and an ethoxy at the 6-position of the indazole ring, emerged as a standout with potent anticancer activity against A549 and MCF7 cell lines. Molecular dynamics simulations underscored the stability of ligand-protein complexes, further validating the compound's robust binding affinity within target proteins. The study also delved into the pharmacokinetics and molecular docking analyses, providing a nuanced understanding of the compounds behavior within biological systems and their binding mechanisms.
Noteworthy antioxidant potential in 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) assays, with compound 4b showcasing the highest antioxidant activity has been observed. Furthermore, the compound 4b that was synthesized demonstrated wide-spectrum antimicrobial efficacy against both gram-negative ones and gram-positive microorganisms. Compound 4c showed potential antifungal activity against Candida albicans and R. oryzae respectively. This integrative study not only successfully synthesized a newer series of indazole derivatives but also provided a holistic understanding of their biological activities.
The SAR studies revealed significant insights across various activities. Nitro and ethoxy substitutions notably enhanced anticancer potency, with compound 5b displaying exceptional activity. Additionally, bromo substitutions showed diverse effects, impacting both anticancer and antioxidant activities. Moreover, the antibacterial activity was significantly improved in the isomer containing an ethoxy group at the 6th position through the incorporation of electron-withdrawing groups, such as nitro at the ortho position. Moreover, the position of substitutions on the aromatic ring significantly influenced the activity, emphasizing the importance of precise molecular design in optimizing therapeutic potential [3-5].
From the second series compounds 6b, 6c and 6d exhibited outstanding anticancer activity, surpassing the potency of established drugs such as Pazopanib. The SAR evaluation unveiled that nitro substitution at the 2-position and 4-fluoro substitution on the indazole ring contributed to enhance anticancer efficacy. Further compounds 6f and 6n exhibited notable antioxidant activity in DPPH and ABTS assays. In the realm of antimicrobial activity, compounds with 4-substituted benzene rings, such as 6e, 6i and 7b consistently demonstrated superior antibacterial efficacy. Methyl, ethoxy and hydroxy groups played crucial roles, with certain compounds showing broad-spectrum antibacterial activity across various strains. For antifungal activity, compounds 7c, 6d and 6f consistently displayed high potency against C. albicans and R. oryzae. SAR analysis emphasized the significance of subtle structural changes in influencing antifungal efficacy. The study also delved into molecular docking and dynamics simulations, providing insights into the compounds stability and interactions with target enzymes [4,5].
The multifaceted approach of synthesis, molecular docking, molecular simulation and extensive characterization positions these compounds as promising candidates for anticancer, antibacterial, antifungal and antioxidant therapies.
Synthetic scheme for series III
In this innovative study, a third newer series of 5-nitro-N-phenyl-3-(phenylamino)-1H-indazole-1-carboxamide derivatives (5'a-5'v) was synthesized and comprehensively characterized (Figure 2). The compounds underwent extensive biological evaluation, including anticancer, antioxidant and antimicrobial assays. In case of anticancer activity, compounds 5'k and 5'n demonstrated substantial activity comparable to established drugs Pazopanib and Doxorubicin, with compound 5'j standing out as a significant contender against A549 and MCF7 cell lines. Compounds 5'k, 5'j and 5's exhibited remarkable antioxidant activity against DPPH and ABTS assays, adding another dimension to their multifaceted therapeutic potential.
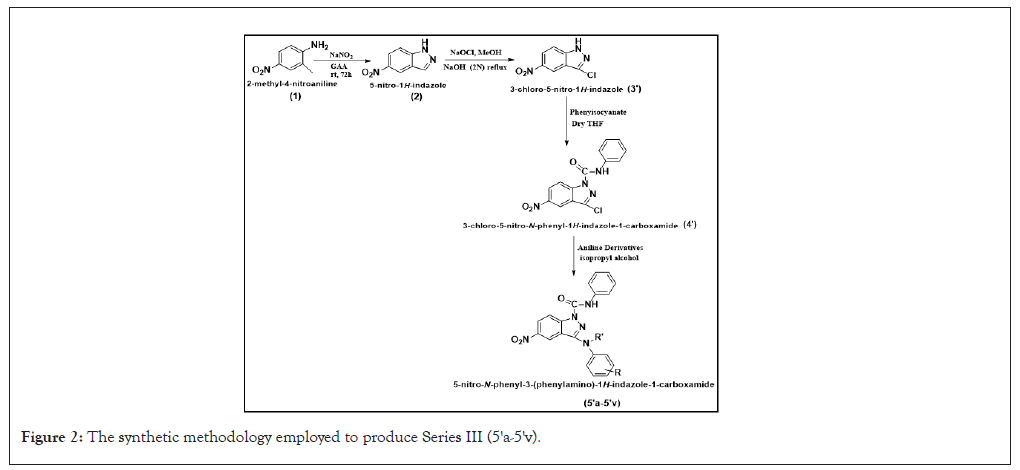
Figure 2: The synthetic methodology employed to produce Series III (5'a-5'v).
Compound 5'b, identified as 3-(methyl(phenyl)amino)-5-nitro-N-phenyl-1H-indazole-1-carboxamide, showcased exceptional antibacterial efficacy across a broad spectrum of bacteria. Noteworthy antifungal potential was observed in compounds 5'k, 5'p and 5'q against R. oryzae and C. albicans respectively. The long-term stability of an extremely potent constituent within the binding site was verified by molecular dynamics simulations, which contributed to the advancement of knowledge regarding its behavior in biological systems.
SAR analysis revealed that compounds with m-methoxy, p-methoxy, and p-bromo aniline derivatives (5'j, 5'k, 5'n) displayed superior anticancer activity, with 5'j (3-OCH3) showing the most potent effect. Conversely, certain substituents like butyl or ethyl groups and some chloro-substituted compounds negatively impacted anticancer activity. Additionally, para-substituted compounds, especially 5'b and 5'c, displayed potent antibacterial activity against Bacillus subtilis, influenced by electronic and steric properties. For antifungal activity, compounds with electron-rich substituents positioned favorably on the aromatic ring demonstrated stronger interactions and efficacy against fungal strains. This study underscored the favourable therapeutic potential of 5-nitro-N-phenyl-3-(phenylamino)-1H-indazole-1-carboxamide derivatives, providing insights into the correlation between diverse aniline derivatives and the 3rd position of the indazole moiety, preparing for further exploration and potential clinical translation [6,7].
Graphical representation of SAR
In our endeavour to comprehensively summarize the SAR across three pivotal activities-anticancer (Series I-III), antioxidant (Series I-III), and antimicrobial (Series I-III), distinct graphical representations have been employed here. Figure 3, meticulously delineates the SAR for anticancer activity with substitutions on the indazole ring, the introduction of a 2-NO2 group displayed significant anticancer activity, while changing the position of the nitro group from ortho to para resulted in a decline in anticancer properties (Figure 3).
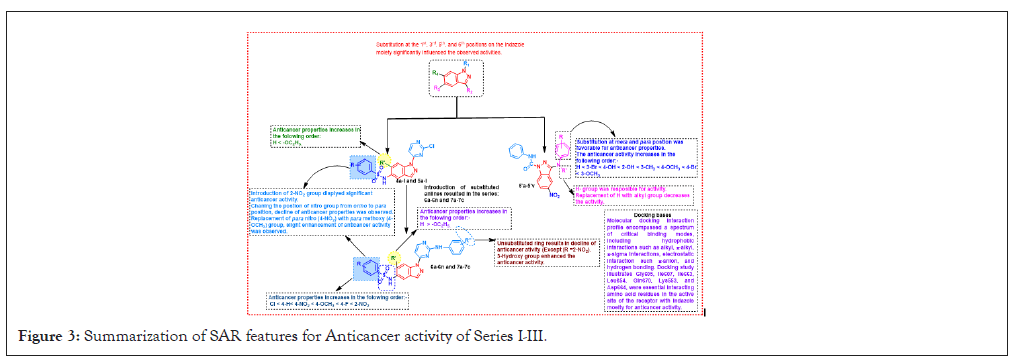
Figure 3: Summarization of SAR features for Anticancer activity of Series I-III.
Notably, the unsubstituted ring resulted in a decline of anticancer activity. Moreover, the presence of a 3-OH group enhanced the anticancer activity in this comparative SAR analysis. Figure 4, offered a detailed insight into the SAR pertaining to antioxidant activity (Figure 4). Introduction of NO2, -OCH3, and F groups contributed to antioxidant activity, with 4-NO2 and 2-NO2 displaying the most potent properties. Changing the position of the nitro group from ortho to para results in a 1.2-fold increases in antioxidant properties. Unsubstituted rings decline antioxidant properties. Finally in Figure 5, presented a comprehensive overview of the SAR for antimicrobial activity, introduction of 2-NO2 showed potent activity towrds S. aureus, B. subtilis, P. aeruginosa, E. coli changing the position of the nitro group from ortho to para resulted in potent potential towards C. albicans, R. oryzae. Introduction of 4-OCH3 or 2-Br increased the activity against C. albicans, R. oryzae. Substitutions with H, Cl, CH3, OH, and F groups alter activity (Figures 5 and 6).
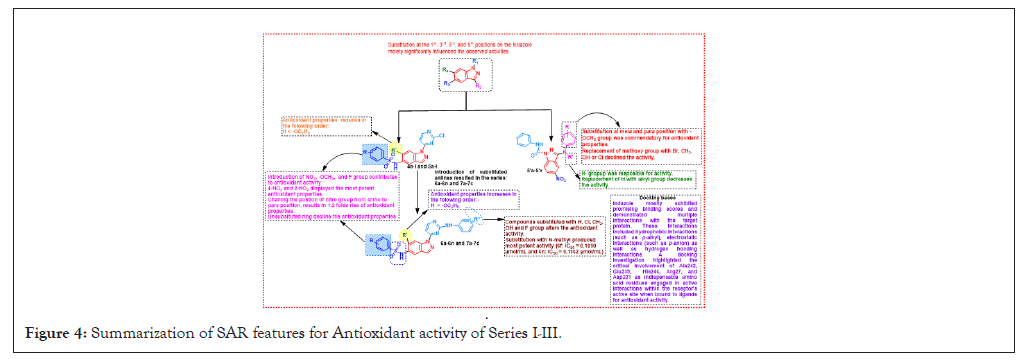
Figure 4: Summarization of SAR features for Antioxidant activity of Series I-III.
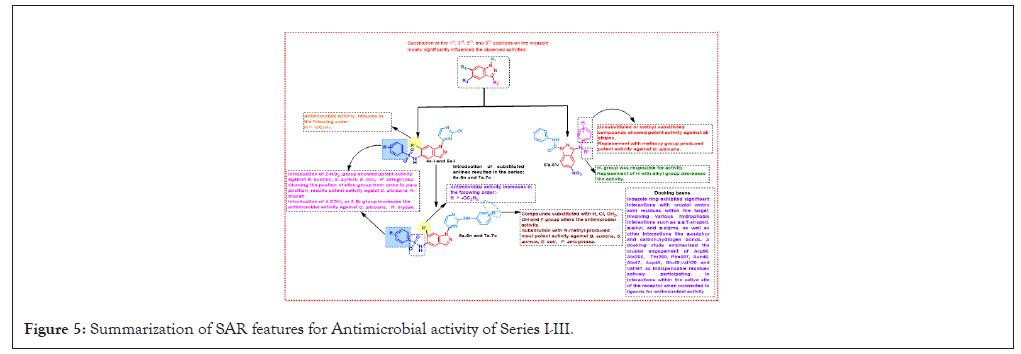
Figure 5: Summarization of SAR features for Antimicrobial activity of Series I-III.
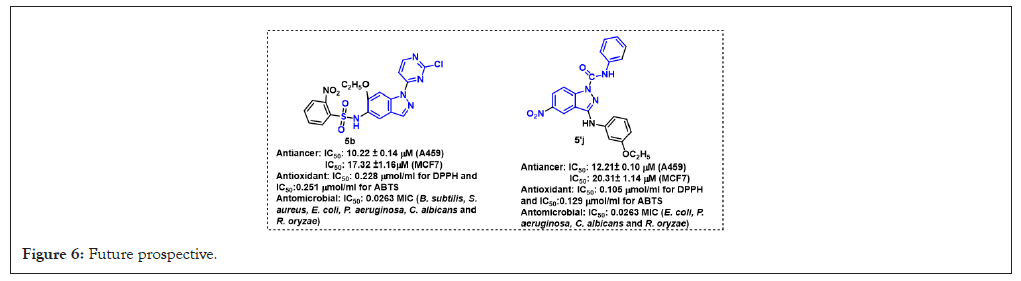
Figure 6: Future prospective.
The remarkable anticancer, antioxidant and antimicrobial activities exhibited by compounds 5b from the first series and 5'j from the third series present promising avenues for future exploration and development.
These compounds demonstrated a multi-targeted approach, suggesting their potential as effective therapeutic agents against a range of diseases. Their ability to target multiple pathways associated with cancer, oxidative stress and microbial infections enhances their utility and efficacy in combating complex medical conditions.
Moving forward, conducting in vivo studies to further evaluate the efficacy and safety profiles of these compounds in animal models are imperative. These studies will provide valuable insights into their pharmacokinetic and pharmacodynamics properties, as well as their potential toxicity and side effects.
In addition, exploring potential synergistic effects of these compounds with existing therapies could lead to the development of combination treatments that offer enhanced therapeutic outcomes while minimizing adverse effects. Overall, the compelling biological activities demonstrated by compounds 5b and 5'j highlighted their potential as lead candidates for further preclinical and clinical development. By using their multitargeted properties and conducting rigorous research, we can strive towards the development of novel therapeutic interventions that address the unmet medical needs of patients suffering from cancer, oxidative stress-related disorders, and microbial infections.
Indazole derivatives have gained significant attention in medicinal chemistry due to their versatility and potential therapeutic applications. This study investigated the design, synthesis and biological evaluation of newer indazole derivatives, aiming to explore their efficacy across multiple therapeutic domains, particularly anticancer, antioxidant and antimicrobial properties. Series III emerged as the most encouraging series, demonstrating superior biological performance across all three activities-anticancer, antioxidant and antimicrobial. This underscores the indazole scaffold's potential for developing novel multifunctional therapeutic agents. The comprehensive SAR analysis highlights the importance of precision design in optimizing biological activity. By carefully selecting and positioning substituents on the indazole ring, researchers can fine-tune the properties of these derivatives to target specific biological pathways and maximize their therapeutic efficacy. Future research will focus on further optimization of the indazole derivatives, including exploring new substituents, synthesizing more complex derivatives and conducting in vivo studies to evaluate their preclinical efficacy in animal models. The findings of this study for the development of novel and effective therapeutic agents based on the indazole scaffold.
Citation: Kapoor A, Yadav M (2024). Design, Synthesis and Biological Evaluation of Derivatives Bearing Indazole Scaffold. J Theor Comput Sci. 10:226.
Received: 06-Nov-2024, Manuscript No. JTCO-24-34965; Editor assigned: 08-Nov-2024, Pre QC No. JTCO-24-34965 (PQ); Reviewed: 25-Nov-2024, QC No. JTCO-24-34965; Revised: 02-Dec-2024, Manuscript No. JTCO-24-34965 (R); Published: 09-Dec-2024 , DOI: 10.35248/2376-130X.24.10.226
Copyright: © 2024 Kapoor A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.