Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2017) Volume 8, Issue 4
The plant extract revealed the presence of phytochemicals such as Phlobatannins, phenols, leucoanthocyanins, saponins, emodins, coumarins and quinones. Process of extraction of pure compound using column chromatography. The gradient of solvent eluted fraction has in pure form, tested and partial characterized. Thin layer chromatographic study was carried out by using various solvent system of varying polarity of which water, ethyl acetate and propanol system suited the best. In vitro anti-inflammatory activity was evaluated using albumin denaturation (50%) membrane stabilization assay (75%) and proteinase inhibitory activity (33.33%). For anti-inflammatory activity Aspirin (85.67%) used as standard drug. Using alfa amylase inhibition assay, In vitro antidiabetic activity was determined, fraction 5 (89.12%) and fraction 6 (80%) were showed at conc. 500 μg/mL. Antimicrobial efficiency of the plant extract fractions was determined using well diffusion method against Pseudomonas sp., Bacillus sp., Protease and Staphylococcus aureus, of which no activity was observed against Pseudomonas sp.
Keywords: Bioprospecting; Terminalia arjuna; Thin layer chromatography; Phytochemical analysis; Anti-inflammatory; Antidiabetic; Antimicrobial
Terminalia arjuna has been reported medicinal value plant for wide applications. The various parts have been reported for health benefit effects such as bark extract injection had increase the coronary flow in heart preparation of rabbit [1]. The bark powder also reported the anti hypocholesterolemic and anti-oxidant effects in human [2] while the methanol extract of T. arjuna leaves have moderate activity against Aspergillus flavus. There is antibiotically resistant strains of microbial pathogens, such as methicillin resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumonia is a problem and so search for better to develop new antimicrobial compounds are continued [3]. Rather than conventional antibiotics, the medicinal plants originated antimicrobial compounds may inhibit bacteria through different mechanisms and it has clinical values against resistant microbes had reported [4]. Modern research has discovered that Terminalia arjuna has antioxidant properties and may be clinically helpful in cardiovascular health. It has antibacterial [5] antimutagenic, hypolipidemic, antioxidant and hypocholesterolemic and antiinflammatory effects. The aim of the present study was to deliver the literal studies of T. arjuna with its phytochemical and pharmacological characteristics. Arjuna regulates cholesterol by decreasing LDL levels in the liver and to be a natural liver tonic. Still today there is no single drug which showed definite and reliable protection or cure from atherosclerotic cardiovascular disorders.
The present paper aims to review Arjuna pharmacognostical, phytochemical, pharmacological, insecticidal, anthelmintic, immunomodulatory, antidiabetic and antioxidant Properties. There is ample evidence of its beneficial effect in coronary artery disease. The extracts of Arjuna used in strengthening the heart muscles, relieving stress, and hypertension. Arjuna is effective for a variety of heart related conditions like high blood pressure, heart palpitations, rapid heartbeat and high cholesterol. These reports are very encouraging and indicate that this should be studied more extensively for its therapeutic benefits.
Arjuna is a good hepatitis reliever and gives heart strength for human. Leaves of arjuna are best for recovery from hepatitis C and also ideal tonic for heart disease. Glucoside reported in the bark of Arjuna is good for heart problem. Arjuna bark powder is also used on broken bone can joint of the fractured bone and also good remedy for swelling of gum and mouth. Bark is effectively used for controlling diarrhoea and dysentery. Arjuna bark is described as heart tonic and herbal preparations is used for treatment of cardiac disorders. World Health Organization, stated that the medicinal plants are best source of variety of drug [6].
Tannins, alkaloids, carbohydrates, terpenoids, steroids, flavonoids and phenols are present in medicinal plant. These bioactive substances of organic compounds play important role in body physiology of human. Secondary metabolites of plant are chemically and taxonomically extremely diverse compounds with unique function. They are widely used in the human therapy. We have extracted and purified these compounds using column chromatography and fractions are collected analyzed for purity and in vitro different assay condition of potency, isolated pure compounds have carried out.
Preparation of extract
1. Freshly collected Plant leaf sample were air dried at room temperature for 5-6 days.
2. Dried leaf sample was grinned to powder using mechanical grinder.
3. Dried leaf powder was homogenized in 10 mL (100%) methanol and was extracted on a rotatory shaker in a centrifuge tube at 350 rpm overnight [7].
4. Crude extract was filtered through Whatman No.1 filter paper and evaporated on steam bath at 30 °C for 30 min (Figure 1).
5. Extract was stored at -20 °C for further study [8].
Column chromatography and TLC
1. Column chromatography was performed according to Patra et al. [9]. The solvent system that exhibited the best separation of compound was chosen for column chromatography. The methanol extract of Terminalia arjuna was absorbed onto silica gel.
2. The column 2 cm × 25 cm was packed with a solution of silica gel with water using the wet slurry method. This involve preparing a solution of silica gel with water in this case, in a beaker and subsequently adding this into the column till it is about three-fourth filled.
3. The solution was stirred for dispersal and quickly added to the column after the gel settles, this method was used to prevent the trapping of air bubble.
4. A boll of wool (glass wool) was pushed into the column to settle a top the packed silica gel.
The solvent system of water: ethyl acetate: propanol (2:5:3) was poured continuously into the column and allowed to drain and about 12 fractions of 5-6 mL was collected in sterile centrifuge tube. The fraction eluted on column was tested with same solvent system by TLC for the presence of active compounds. The column eluted fractions are lyophilized dried as power and stored at -20 °C (Figure 2).
Thin Layer Chromatography (TLC) [10] was performed
1. Fraction eluted on column was subjected to TLC as per conventional one-dimensional ascending method using silica gel (60F254 MERCK) pre-coated plate, plate was cut with ordinary household scissor.
2. Plate marking were made with soft pencil.
3. Glass capillary were used to spot the sample.
4. For TLC applied sample volume 1 μL by using capillary and solvent system was used is water: ethyl acetate: propanol.
5. After pre-saturation with mobile phase 20 min for development of band were used.
6. After running the plates, they are dried using dryer and plates were observed under various wavelength at 254 nm and 366 nm for band detection.
7. Colour of the spot and pattern were observed and RF value were calculated using formula:

Phytochemical screening
Phytochemical studies were carried out for methanol extracts of Terminalia arjuna leaves to detect the presence of different phytochemicals (Table 1).
| S. No. | Phytochemicals | Test | Inference | Observation |
|---|---|---|---|---|
| 1 | Alkaloid | Add 2 mL of extract to 2N HCL decand aqueous layer formed and few drops of mayers reagent | Cream precipitate observe indicating the presence of alkaloid | Cream precipitate was not observed |
| 2 | Phenolic compounds | Compounds-Add 3-5 drops of 5% FeCl3 solution to 2 mL of extract | Formation of deep blue colour | Deep blue colour was observed |
| 3 | Flavonoids | In 2 mL of extract, add 2-5 drops of 1N NaOH | Formation of yellow orange colour | Yellow orange colour was not observed |
| 4 | Saponins | Add 2mL of extract with 6 mL of water in a test tube | Observe for persistent foam | Observation of persistent foam |
| 5 | Tannins | Add 2mL of aqueous extract with 2mL of distilled water and few drops of FeCl3 | Formation of green precipitate | Green precipitate was observed |
| 6 | Leucoanthocyanin | Add 5 mL of aqueous extract to 5mL of isoamyl alcohol | Upper layer appears red in colour | Red colour was observed |
| 7 | Quinones | Add 2 mL of extract with concentrated HCl | Formation of yellow precipitate | Yellow precipitate was observed |
| 8 | Coumarin | Add 3mL of 10% NaOH to 2mL of aqueous extract | Formation of yellow colour | Yellow colour was observed |
| 9 | Steroid | Dissolve 1mL of extract in 10mL of chloroform and add equal volume of concentrated H2SO4 | The upper layer turns red and H2SO4 layer shows yellow green fluorescence | The upper layer was not red and H2SO4 layer was not yellow green fluorescence |
| 10 | Emodins | Add 2 mL of extract with concentrated HCl | Formation of yellow precipitate | Yellow precipitate was observed |
| 11 | Phlobatanin | Add 2 mL of aqueous extract to 2 mL of 1% HCl and boil the mixture | Deposition of red precipitate | Red precipitate was observed |
| 12 | Anthocyanin | Add 2 mL of aqueous extract to 2 mL of 2N HCl and Ammonia | Appearance of pink- red turns Blue- violet | Pink-red colour turns Blue-violet was not observed |
Table 1: Phytochemical tests for secondary metabolites present in extract of Arjun.
Estimation of protein content of eluted fractions of column chromatography using nano drop spectrophotometer
The protein content was measured using nanodrop, casein used as standard for estimation of protein Anti-inflammatory Activity was performed according to Leelaprakash and Dass [11].
Albumin denaturation assay
1. Method of Mizushima and Kobayashi and Sakat et al. followed by minor modification [12,13].
2. The reaction mixture was consisting of test extract and 1% aqueous solution of bovine albumin fraction, pH of the reaction mixture was adjusted using small amount of 37 °C HCL
3. The sample extract was incubated at 37 °C for 20 min. and then heated to 51 °C for 20 min
4. After cooling the sample, the turbidity was measured spectrophotometrically at 660 nm
5. The experiment was performed in triplicate
6. Percent inhibition of protein denaturation was calculated as follow
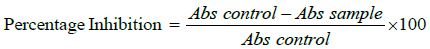
Preparation of red blood cells (RBCs) suspension:
1. Fresh whole human blood 10 mL was collected and transferred to the centrifuge tube.
2. The tubes were centrifuged at 3000 rpm for 10 min. and were washed three times with equal volume of normal saline.
3. The volume of blood was measured and constituted as 10% v/v suspension with normal saline [13,14].
Heat induced haemolytic:
1. The reaction mixture 2 mL consisted of 1 mL of test sample solution and 1 mL of 10% RBCs suspension
2. Instead of test sample only saline was added to the control test tube.
3. Aspirin was taken as a standard drug.
4. All the centrifuged tube containing reaction mixture were incubated in water bath at 56 °C for 30 min
5. At the end of the incubation the tube was cooled under running tap water
6. The reaction mixture was centrifuged at 2500 rpm for 5 min. and the absorbance of the supernatants was taken at 560 nm
7. The experiment was performed in triplicate for all the test sample, % membrane stabilization activity was calculated by formula [13,15].
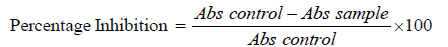
Protein inhibitory action:
1. The test was performed according to the modified method of Oyedepo et al. and Sakat et al. [13,16].
2. The reaction mixture (2 mL) was containing 0.06 mg trypsin, 1 mL of 20 Mm Tris HCL buffer (pH 7.4) and 1 mL test sample of different concentration.
3. The reaction mixture was incubated at 37 °C for 5 min. and then 1 mL of 0.8% (W/V) casein was added
4. The mixture was inhibited for an additional 20 min., 2 mL of 70% perchloric acid was added to terminate the reaction
5. Cloudy suspension was read at 210 nm against buffer as blank
6. The experiment was performed in triplicate
7. Percentage protein inhibition activity was calculated by formula
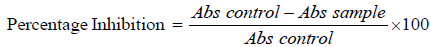
Antidiabetic activity was performed according to Dhritiv et al. [17].
Inhibition of alpha amylase enzyme:
Standard maltose curve:
1. 0.2-1 mL of standard maltose (1 mg/mL) was taken into different tube.
2. Make the volume to 1 mL in each case with distilled water.
3. Added 1 mL of DNSA (Dinitro salicylic acid) reagent to each tube and then place all the tubes in boiling water bath for 15 mins.
4. Add 8 mL of distilled water in each tube and mix the content.
5. Then read the absorbance of the solution in Calorimeter at 570 nm against blank solution.
Alpha amylase inhibition assay:
1. 100-500 μL of extract was taken into different test tubes, make the volume 0.5 mL with phosphate buffer of pH 6.8
2. Blank was measured by taking 1 mL of phosphate buffer
3. Control was measured by taking 0.5 mL of phosphate buffer
4. The solution was taken treated with 0.5 mL of alpha amylase (0.5 mg/mL)
5. The solution was incubated at 25 °C for 10 mins
6. Added 0.5 mL of 1% starch solution in 0.02 M Sodium phosphate buffer of pH 6.9 to all tubes and then incubated at 25 °C for 10 mins
7. The reaction was stopped by DNSA and the reaction mixture was kept in boiling water bath for 5 mins cooled to RT
8. The solution was mixed with 8 mL distilled water
9. Read the absorbance of the solution in calorimeter at 570 nm against blank solution
10. Amount of maltose produced is calculated using standard maltose curve and enzyme activity is calculated by using formula
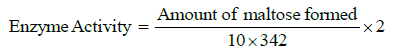
Antimicrobial activity was performed according to Narendra et al. [18].
Microorganism used
The bacterial strains were collected from microbial culture collection laboratory VSBT
1. The antimicrobial activity was performed by agar well diffusion method for solvent extract.
2. 20 ml of media Muller and Hinton (MH) agar was poured into the petri plates along with inoculum.
3. A well was prepared in the plate with the help of cork bores (6 mm).
4. 20 μL of the test sample was poured in each well using sterile micropipette.
5. For positive control standard, antibiotic tetracycline (30 mcg) was used.
6. The plates were incubated overnight at 37 °C in BOD incubator.
7. The microbial growth was determined by measuring the diameter of zone of inhibition.
8. The entire process was carried out aseptically in the laminar air flow.
Column chromatography and TLC studies
Thin layer chromatographic studies of partial purified methanol fraction of Terminalia arjuna was done by using silica gel 60 F254 (MERCK) aluminium plate. Solvent system water: ethyl acetate: propanol (2:5:3) was used for separation of compound. Partial purified fraction eluted on column chromatography showing different band pattern at 254 nm and 366 nm (Figure 3) Spot were characterized by Rf value under UV light (Figure 4).
Phytochemical analysis
Qualitative phytochemical investigation of extract of Terminalia arjuna leaves showed the presence of saponins, coumarins, emodins, phenols, quinones, leucoanthacyanins and phlobatannins. Methanolic extract of Terminalia arjuna leaves contain maximum number of phytoconstituents. The results of phytochemical screening and qualitative analysis were showed in the Tables 2-4 (Figures 5 and 6).
| Fraction No. | Solvent system | No. of spot detected | Rf value | ||
|---|---|---|---|---|---|
| 254 nm | 366 nm | 254 nm | 366 nm | ||
| 1 | Water: ethyl acetate: propanol | - | - | - | - |
| 2 | Water: ethyl acetate: propanol | 1 | 1 | - | - |
| 3 | Water: ethyl acetate: propanol | 1 | 1 | - | - |
| 4 | Water: ethyl acetate: propanol | 1 | 1 | 0.60 | - |
| 5 | Water: ethyl acetate: propanol | 1 | 1 | 0.58 | - |
| 6 | Water: ethyl acetate: propanol | 1 | 1 | 0.85 | - |
| 7 | Water: ethyl acetate: propanol | 1 | 1 | 0.67 | - |
| 8 | Water: ethyl acetate: propanol | 1 | 1 | 0.82 | - |
| 9 | Water: ethyl acetate: propanol | 1 | 1 | 0.59 | - |
| 10 | Water: ethyl acetate: propanol | 1 | 1 | 0.40 | - |
| 11 | Water: ethyl acetate: propanol | 1 | 1 | - | |
| 12 | Water: ethyl acetate: propanol | 1 | 1 | --- | - |
Table 2: TLC investigation and banding pattern for column eluted fractions.
| Fraction no | Protein concentration µg /mL |
|---|---|
| 1 (solvent eluted) | -1.058 |
| 2 (solvent eluted) | 48.788 |
| 3 (solvent eluted) | 107.445 |
| 4 (solvent eluted) | 106.418 |
| 5 (solvent eluted) | 11.308 |
| 6 (solvent eluted) | 4.89 |
| 7 (solvent eluted) | 1.216 |
| 8 (solvent eluted) | 1.418 |
| 9 (solvent eluted) | 2.834 |
| 10 (solvent eluted) | 5.535 |
| 11 (solvent eluted) | 3.508 |
| 12 (solvent eluted) | 1.232 |
Table 3: Determination of Protein concentration by Nano drop technique.
| Name of the phytochemical | Result | Partially purified fraction No. 4, 6, 7 and 8 |
|---|---|---|
| Phenolic compounds | + | + |
| Phlobatannins | + | + |
| Alkaloids | - | - |
| Saponins | + | - |
| Flavonoids | - | - |
| Coumarins | + | + |
| Anthocyanins | - | - |
| Terpenoids | + | + |
| Steroids | - | - |
| Fatty acid | - | - |
| Emodins | + | + |
| Quinones | + | + |
Table 4: Phytochemical constituents present in methanol extracts of Terminalia arjuna leaves and partially purified fraction.
In vitro anti-inflammatory activity
Albumin denaturation assay: Denaturation of protein is a welldocumented cause of inflammation. As a part investigation on the mechanism of the anti- inflammatory activity, ability of fraction inhibit denaturation was studied. It was effective in inhibiting head induced albumin denaturation. Maximum inhibition was observed in fraction No 4 and 8 as shown in Table 5. The percentage of albumin denaturation inhibition is given of respective column eluted fractions (Figure 7).
| Test sample | Albumin Denaturation |
|---|---|
| Fraction 4 | 50 ± 0.003 |
| Fraction 6 | 13.33 ± 0.003 |
| Fraction 7 | 13.33 ± 0.003 |
| Fraction 8 | 33.33 ± 0.003 |
| Aspirin | 76.54 ± 0.003 |
Table 5: Percentage inhibition of Albumin denaturation assay.
Membrane stabilization assay
The HRBC membrane stabilization has been used as a method to study the in vitro anti-inflammatory activity because the erythrocyte membrane is analogous to the lysosome membrane and its stabilization implies that the column eluted fraction may well stabilize lysosomal membranes. Stabilization of lysosomal is important in limiting the inflammatory response by preventing the release of lysosome constituents of activated neutrophil, such as bacterial enzymes and proteases, which causes further tissue inflammation and damage upon extra cellular release. The lysosomal enzymes released during inflammation produce a various disorder. purified fraction was effective in membrane stabilization at different concentration as shown in Table 6, maximum inhibition of fraction no.4 and 8 (75%) was observed at 500 μg/mL, followed by fraction 6 (50%) and fraction no.7 (50%) aspirin a standard drug shows maximum inhibition 81.32% at conc.500 μg/mL. Membrane stabilization assay of partially eluted sample was shown in the Figure 8.
| Test Sample | Membrane Stabilization assay |
|---|---|
| Fraction 4 | 75 ± 0.004 |
| Fraction 6 | 50 ± 0.004 |
| Fraction 7 | 50 ± 0.004 |
| Fraction 8 | 75 ± 0.004 |
| Aspirin | 81.32 |
Table 6: Percentage inhibition of membrane stabilization assay.
Proteinase inhibitory activity
Proteinases have been implicated in arthritic reactions. Neutrophils are known to be a rich source of proteinase which carries in their lysosomal granules many serine proteinases. It was previously reported that leukocytes proteinase play an important role in the development of tissue damage during inflammatory reactions and significant level of protection was provided by proteinase inhibitors. Terminalia arjuna partial purified fraction exhibited significant anti proteinase activity at different concentrations as shown in Table 7. Maximum inhibition of fraction no.6 and 7 (33.33%) was observed at 500 μg/mL, followed by fraction 4 (25%) and fraction 8 (25%) aspirin a standard drug shows maximum inhibition 85.67% at conc.500 μg/mL. The protein denaturation of column eluted sample was shown in the Figure 9.
| Test sample | Proteinase inhibition |
|---|---|
| Fraction 4 | 25 ± 0.004 |
| Fraction 6 | 33.33 ± 0.004 |
| Fraction 7 | 33.33 ± 0.004 |
| Fraction 8 | 25 ± 0.004 |
| Aspirin (Control) | 85.67 |
Table 7: Percentage inhibition of Proteinase denaturation of column eluted fractions of Terminalia arjuna.
In vitro antidiabetic activity
Alpha amylase inhibition assay: The intestinal digestive enzyme alpha-amylase plays vital role in the carbohydrate digestion. Antidiabetic therapeutic approach reduces the post prandial glucose level in blood by the inhibition of alpha- amylase enzyme. The in vitro alpha amylase inhibitory studies demonstrated that Terminalia arjuna has well antidiabetic activity. Partially purified fraction showed maximum inhibition of fraction No.5 (84.98%) at concentration 500 μg/mL and fraction no.6 (70.71%) at concentration 500 μg/mL. conc. Dependent percentage inhibition listed in Table 8 (Figures 10 and 11).
| Conc µg/mL | % Fraction 5 | % Fraction 6 | % Standard n | |||
|---|---|---|---|---|---|---|
| Abs. | % inhibition | Abs. | % inhibition | Abs. | % inhibition | |
| 100 | 0.186 | 69.12 | 0.044 | 55.8 | 0.027 | 67 ± 0.04 |
| 200 | 0.994 | 77.36 | 0.056 | 59.67 | 0.032 | 68 ± 0.04 |
| 300 | 1.026 | 85.08 | 0.658 | 34.24 | 0.053 | 81.42 ± 0.05 |
| 400 | 1.165 | 57.89 | 0.12 | 68.7 | 0.057 | 78.35 ± 0.05 |
| 500 | 0.426 | 89.12 | 0.128 | 75.08 | 0.075 | 80 ± 0.06 |
Table 8: In vitroalpha amylase inhibition method.
Antimicrobial activity
Antimicrobial activity of methanol extract of Terminalia arjuna leaves gave different zone of inhibition against the organisms tested. The extract showed antimicrobial activity against Gram +ve and Gram -ve bacterial strains as shown in Figure 12. Fraction No. 3, 4 and 6 of methanolic extract of Terminalia arjuna was most sensitive against Staphylococcus aureus, Protease mirabilis and Bacillus subtilis with maximum zone of inhibition of diameter 15 mm, 12 mm, 15 mm respectively at conc. of 20 μg as shown in Table 9. Methanolic extract did not show resistance against Pseudomonas aeruginosa.
| Fraction No. 20 µg/50 µL |
Staphylococcus aureus | Protease mirabilis | Bacillus substilis | Pseudomonas aeruginosa |
|---|---|---|---|---|
| Fraction 4 | 15 mm | _ | _ | _ |
| Fraction 9 | _ | 12 mm | _ | _ |
| Fraction 6 | _ | _ | 15 mm | _ |
| Fraction 7 | _ | _ | 14 mm | _ |
Table 9: Antimicrobial activity Terminalia arjunaby well diffusion method.
Qualitative phytochemical investigation of extract of T. arjuna leaves showed the presence of alkaloids, aponins, coumaris, emodins, phenols, quinones, leucoanthacyonins and phlobatannins. A total of 12 different fractions were obtained from the extract of T. arjuna leaves through column chromatography. The most suitable solvent system out of the three-different solvent system was Water: ethyl acetate: propanol (2:5:3) solvent system, which provided visible bands in fraction 4 and 5 only. The anti-inflammatory activity was most effectively depicted by fraction 4, 6, 7 and 8 in the respective tests conducted. The antidiabetic activity was highest in fraction 5 and 6 at a concentration of 500 μg/mL. Methanolic extract of T. arjuna was most sensitive against Staphylococcus aureus, Protease mirabilis and Bacillus subtilis except Pseudomonas aeruginosa.
In vitro assay of the extract of arjuna plant showed the various activities such as anti-inflammatory was valuated using albumin denaturation, membrane stabilization assay and proteinase inhibitory activity. In vitro antidiabetic activity was determined, fraction no 5 (89.12%) and fraction no 6 (80%) of the pure compound isolated using column chromatography techniques. These compounds showed pure band on TLC plate. Further identification of compounds and its structure need to be identified. These active Phyto constitutes would study further for targeted various spectrum of diseases and will determine its physiological and metabolic effects in animal’s model, aspects of preclinical studies towards drug development process. Several medicinal values of the reported of genus Terminalia [19]. The preliminary phytochemical evaluation of flavonoids and alkaloids was carried out [20]. As the arjuna is reported historical medicinal value and safe for consummation so trials will be low risk. The fraction no S6 and S7 showed good antimicrobial activities against Staphylococcus aureus and Bacillus substilis so the active component may be used as bactericidal agent.
On the basis of results, the following major conclusions were drawn. The presence of seven Phytochemicals in T. arjuna confirms its antioxidant activity. The results of column chromatography and thin layer chromatography enabled the purification of T. arjuna leaves extract. The anti-inflammatory effect of four fractions concludes the anti-inflammatory property of Terminalia arjuna and good antidiabetic property which was confirmed by the anti-diabetic activity of two fractions obtained. Terminalia arjuna extracts has shown good activity against both the gram positive and gram-negative bacteria.
However, further research is required to determine the compound that has contributed to these activities of the extract and its exact mode of action.