Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2023)Volume 13, Issue 3
Recent studies have demonstrated that vitamin D insufficiency is common in tropical countries. Vitamin D is a fat-soluble vitamin that is obtained by sunlight exposure, diet, or supplements and it is an important factor influencing the health status of individuals. Vitamin D is naturally present in the mushroom and once exposed to a source of Ultraviolet (UV) radiation, can generate nutritionally relevant amounts of vitamin D. Hence, the purpose of this study is to determine, fractionalised and quantify the amount of vitamin D in the oyster mushroom (Pleurotus ostreatus). An amount of fine powder mushroom was weighed and incubated with 20 ml hexane and 50% potassium hydroxide mixture for 10 minutes at 37°C. The mixture solvent was then centrifuged for 45 minutes at 2000 rpm before the supernatant was stored at -20°C for a week until a white precipitate was formed. Later, it was diluted with 95% methanol and injected into the recycling preparative High-Performance Liquid Chromatography (HPLC) system. The system was operated at a flow rate of 20 ml/min with a running time of 50 minutes. The retention time for vitamin D peak was recorded at 32 minutes. The eluent was collected at this particular time before the quantification of vitamin D was performed by using Ultra High-Performance Liquid Chromatography (UHPLC) at 265 nm. It was recorded that the concentration of vitamin D in the fractionated solvent was 69.6 ± 7.25 ppm (2784 ± 290 IU). The linearity of this method was found to be within the acceptable recovery limit range (r2>0.999). The recycling preparative HPLC has great potential as a useful tool for the determination and fractionation of vitamin D in food samples. Commercially available vitamin D3 supplements are usually derived from the wool of healthy sheep. Thus, the extraction of vitamin D from mushrooms has great potential as an alternative for vegan, vegetarian and halal conscious consumers.
Vitamin D; Oyster mushroom; Recycling preparative; HPLC; Fractionation; quantification
People are suffering from vitamin D deficiency due to inadequate exposure to sunlight and increased use of sunscreen protection [1]. Vitamin D is a sunshine vitamin that can be recognized as a steroid hormone. Although a large number of tropical countries lie in zones that have sufficient sunlight for vitamin D synthesis for most if not all of the year, recent studies have demonstrated that vitamin D insufficiency is common in tropical countries like Vietnam, Malaysia and Indonesia [2,3]. Thus, vitamin D deficiency is becoming a global health problem. As such, the use of vitamin supplements and foods containing vitamin D is an important source. However, the number of foods that are high in vitamin D is limited. Vitamin D plays an important role in maintaining extracellular fluid concentration calcium and phosphorus homeostasis within a normal range. As an important nutrient for bone strength, this vitamin also helps to prevent rickets in children and osteoporosis in adult, reduce tumor growth as well as lowering risk of cancer. This fat-soluble vitamin was taken for granted as it is assumed to be plenty in daily food. Unfortunately, only a few foods naturally contain vitamin D and others are fortified foods [4]. There are two major form of vitamin D which is vitamin D2-ergocalciferol, derived predominantly from fungi and D3-cholecalciferol, originating from animals source [5,6]. A primary source of vitamin D3 in humans and many animals occurs from the conversion of 7-dehydrocholesterol in the epidermis to vitamin D3 during exposure to Ultraviolet (UV) radiation present in sunlight [7].
Mushrooms are one of the good food sources of vitamin D [8]. Mushrooms exposed to sunlight or UV radiation are an excellent source of dietary vitamin D2 because they contain high concentrations of vitamin D precursor, provitamin D2. The provitamin D2 is efficiently converted to previtamin D2. Once formed, this unstable conformer rapidly isomerizes to vitamin D2 [9] in a similar manner that previtamin D3 isomerizes to vitamin D3 in human skin. Interestingly, mushrooms have been recognized as an important food item for their crucial role in human health [10]. It is also known as a vegetarian’s meat because it is a rich source of protein, vitamins and minerals. Several species of mushrooms are of great importance because of their medicinal properties against hypertension, diabetes and cancer [11]. It was reported that the nutritional and chemical compositions of the mushrooms are responsible for their medicinal values. Hence, the objective of this study is to determine, fractionalise and quantify the amount of vitamin D in the oyster mushroom (Pleurotus ostreatus) that is widely cultivated in Malaysia. The Pleurotus spp. was chosen because it contains high concentrations of vitamin D compared to other mushrooms. Furthermore, the cultivation of Pleurotus spp. is reasonably easy, low cost production technology, and ideally suited for cultivation throughout the year in various regions of the tropical country.
Chemical and reagents
Vitamin D standards (ergocalciferol and cholecalciferol ≥ 98%) were purchased from sigma aldrich (St. Louis, USA) and stored at -20°C, respectively. Potassium Hydroxide (KOH) pellets (≥ 85%) and HPLC grade methanol (purity ≥ 99.9%) was purchased from Merck (Darmstadt, Germany) and stored at 37°C in a safety cabinet.
Raw materials
Fresh oyster mushrooms (Pleurotus ostreatus) were purchased from a local hypermarket in Malaysia. The gills were placed upward to the sunlight for 3 hours. After that, the stem of the mushroom was cut and the mushroom was placed at -20°C overnight. Later, the mushroom was freeze-dried for 48 hours, ground into a fine powder and stored in vacuum tight containers till use.
The extraction process of oyster mushroom
Fifty percent of potassium hydroxide and 3 g of fine mushroom powder were placed in a universal bottle. Later, 20 mL of hexane was added into the bottle and incubated for 10 minutes at room temperature. Then, the mixture was centrifuged for 45 minutes at 2000 rpm. After that, the supernatant was pipetted out and kept at -20°C for a week until the white precipitate appears. Later, the supernatant was dried using a rotary evaporator and nitrogen gas.
Fractionation of oyster mushroom
Approximately, 0.015 g of dried extraction powder was diluted with 4 ml methanol and was filtered through a 0.2 μm membrane filters before injecting the sample into a recycling preparative High-Performance Liquid Chromatography (HPLC) system. The system used a reversed-phase C18 column (30 mm × 250 mm) in an isocratic elution with 100% methanol as a mobile phase. The flow rate was 20 ml/min with a total run time of 50 minutes. The fraction was collected at a specific elution time and subjected to quantification using Ultra High-Performance Liquid Chromatography (UHPLC dionex ultimate 3000 series system) with dionex acclaim polar advantage II reversed-phase C18 column (4.6 mm × 250 mm, 5 μm) at 265 nm.
Statistical analysis
Data were expressed as mean value ± standard deviation. A Statistical Package for Social Science (SPSS) for windows version 23.0 was used to analyse the data.
Determination of vitamin D by recycling preparative HPLC
The amount of vitamin D in oyster mushroom was shown in Table 1. The analysis was conducted in triplicate. The absorbance rate and retention time of vitamin D in oyster mushrooms were compared with the absorbance rate of the standard compound of vitamin D at four different wavelengths (245 nm, 265 nm, 270 nm and 280 nm) to get the best fraction elution (Figures 1-3). Although the best wavelength with the highest peak for vitamin D was recorded at 270 nm, an absorbance rate of 265 nm was chosen based on the official methods for determining vitamin D in food by HPLC with UV detection. In order to determine the accurate retention time for vitamin D, an overlapping chromatogram graph was generated for the oyster mushroom and the standard compound of vitamin D in the same matrix. However, as the diameter of recycling preparative HPLC column JAIGEL-ODS AP30 sp120-16 was larger than the usual column the vitamin D peak from the oyster mushroom looked minuscule in the overlaying chromatogram (Figure 4). Nevertheless, both vitamin D standard compound and the oyster mushroom emerged at the same retention time which was at 32 min. To further elucidate this finding, the UHPLC system was used to compare the result from the normal extraction process without recycling preparative HPLC and the fractionated oyster mushroom with recycling preparative HPLC respectively. The result showed that the vitamin D peak of oyster mushroom that has gone through the fractionation process was discrete, sharp and clear when compared with the normal extraction process of oyster mushroom (Figures 5 and 6) [12,13].
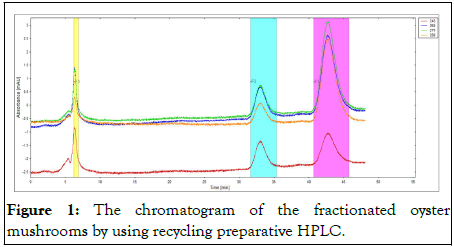
Figure 1: The chromatogram of the fractionated oyster mushrooms by using recycling preparative HPLC.
The fraction was differentiated by four different colour representing four different wavelengths. The fraction was collected in between 30 min-35 min and 40 min-45 min for further analysis with UHPLC. All samples were analysed in triplicate.
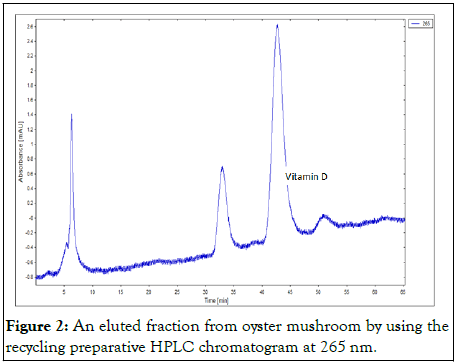
Figure 2: An eluted fraction from oyster mushroom by using the recycling preparative HPLC chromatogram at 265 nm.
Absorbance wavelength of 265 nm was used as recommended by the AOAC official methods for vitamin D.
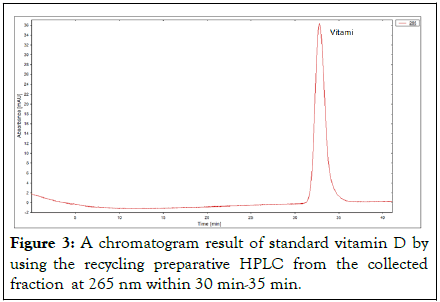
Figure 3: A chromatogram result of standard vitamin D by using the recycling preparative HPLC from the collected fraction at 265 nm within 30 min-35 min.
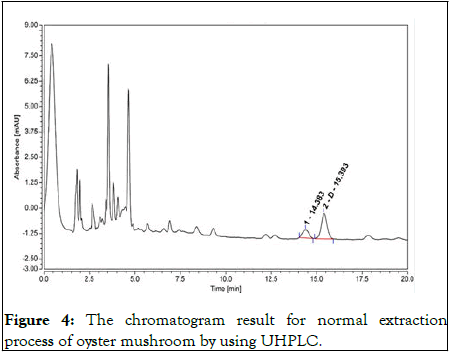
Figure 4: The chromatogram result for normal extraction process of oyster mushroom by using UHPLC.
Many impurities appeared in the normal extraction and vitamin D peak was not discrete at 15 min retention time.
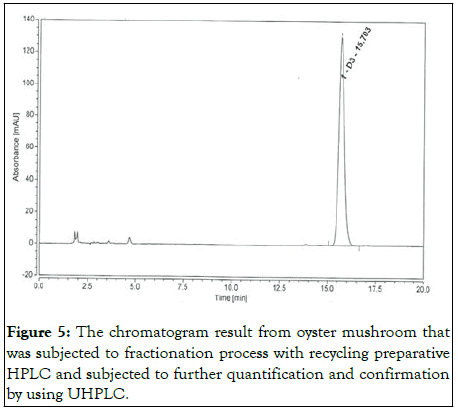
Figure 5: The chromatogram result from oyster mushroom that was subjected to fractionation process with recycling preparative HPLC and subjected to further quantification and confirmation by using UHPLC.
No impurities appeared and vitamin D peak was discrete at 15 min retention time.
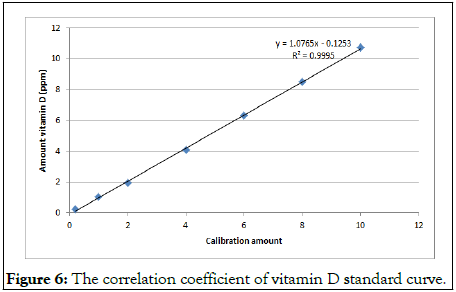
Figure 6: The correlation coefficient of vitamin D standard curve.
Fractionation confirmation of vitamin D using UHPLC: As mentioned previously, the highest concentration of vitamin D in the fractionated solvent of oyster mushroom was quantified at 69.6 ± 7.25 ppm (2784 ± 290 IU) (Table 1) by UHPLC at 265 nm with UV detection. The method showed linearity of detection within the acceptable recovery limit range (r2>0.9995). Although this method was found to be able to eliminate the impurities of vitamin D, the identification and determination of vitamin D in food item has always been a challenge due to the low level of vitamin D and the existence of multiple vitamin D active compounds in the food that further exacerbates this issue.
| Fraction vitamin D | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Mean (ppm) | 5.36 ± 0.02 | 11.36 ± 2.26 | 38.88 ± 0.13 | 69.59 ± 7.25 |
| Vitamin D (IU) | 214.30 ± 0.80 | 454.52 ± 90.40 | 1558.64 ± 5.20 | 2783.62 ± 290.00 |
| *Conversion factor from ppm to IU for vitamin D: ppm/0.025 | ||||
Table 1: Amount of vitamin D from four different fractions of oyster mushroom. All the analysis was conducted in triplicate.
A study done by Keegan and his colleagues on mushroom extract found to contain many types of vitamin Ds other than vitamin D2 and D3. In this study, we reported it as vitamin D, as mushrooms do not only produce vitamin D2 but also vitamin D3 and vitamin D4. In the same study, it was reported that the bioavailability of vitamin D from mushrooms, when compared with the bioavailability of vitamin D2 or vitamin D3 from supplements, revealed the same bioavailability effectiveness by taking vitamin D at 2000 IUs to maintain blood levels of 25-hydroxyvitamin D in the body. Since a significant amount of vitamin D was detected in mushrooms, this food item can become a good source of vitamin D, especially for vegans and vegetarians.
In conclusion, a high amount of vitamin D in oyster mushrooms can be fractionated and quantified using the recycling preparative HPLC and UHPLC. This technique has great potential as a useful and economical tool for mass production of vitamin D because of its precision and rapidness.
The data used to support the findings of this study are available from the corresponding author upon request.
The authors declare that there are no conflicts of interest regarding the publication of this paper. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
We would like to express our gratitude the director general of health, Malaysia for giving permission to publish this manuscript. This project was supported by the national institutes of health, Malaysia (Project code: JPP- IMR: 14-009).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rashed AA, Gunasegavan RDN, Sarbini SR (2023) Determination, Fractionation and Quantification of Vitamin D in Pleurotus ostreatus (Oyster Mushroom) using Recycling Preparative High Performance Liquid Chromatography (HPLC). J Nutr Food Sci. 13:018.
Received: 19-Dec-2022, Manuscript No. JNFS-22-21000; Editor assigned: 21-Dec-2022, Pre QC No. JNFS-22-21000 (PQ); Reviewed: 04-Jan-2023, QC No. JNFS-22-21000; Revised: 27-Mar-2023, Manuscript No. JNFS-22-21000 (R); Published: 03-Apr-2023 , DOI: 10.35248/2155-9600.23.13.018
Copyright: © 2023 Rashed AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.