Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
+44 1478 350008
ISSN: 2161-0398
+44 1478 350008
Research Article - (2024)Volume 14, Issue 4
Acetylation of gum Arabic was achieved using acetic anhydride as solvent. The ester group formed was confirmed by FTIR spectra having absorption band of 750 cm-1-700 cm-1. Viscometric study of the pure and acetylated samples was carried out. Relative viscosity of acetylated gum was found to be higher than that of the pure gum. Intrinsic viscosity was determined for the two samples using different plot methods taking Huggin’s plot as standard. The intrinsic viscosity was found to be 86.43 cm3/g and 64.59 cm3/g for acetylated and pure gum arabic respectively. Relative errors of other methods for the two samples was compared to that of Huggins and the plots that are most comparable to Huggins with relative errors less than 5% are; Martin, Lyon-Tobolsky, Staudinger-Heuer, Maron-Reznik and our proposed method. Our proposed method which was a modification of the Kreiser method gave relative error less than 2%, for both pure and modified gum. Whereas the Kreiser method gave relative error greater than 15 % for both methods. The critical concentration for the samples was found to be 0.0116 g/cm3 and 0.0155 g/cm3 for acetylated and pure gum respectively. This shows that there was no molecule-molecule entanglements during viscosity measurements.
Intrinsic viscosity; Acetylation; Gum Arabic
Gum Arabic exudate is gummy, dry and edible. It is usually obtained from stems and branches of Acacia senegal and Acacia seyal that have high content of non-viscous soluble fibre. It is a salt of complex polysaccharides that is neutral or slightly acidic in nature, containing Ca2+, Mg2+ and K+. Its most distinguishing character among other gums is that it is extremely soluble in water. The exudate is found mainly in unhealthy trees that are affected by diseases, drought or poor nutrition. The gum comes out through wounds carved in the bark of the tree in liquid drops, which then becomes hard with time. The tree’s taxonomic classification is: Genus Acacia, subfamily Mimosoidene and family; Leguminosae.
The gum itself comprise of different materials, but may be separated in to three major parts. 88.4% of the gum is arabinogalactan with 0.35% protein content and has molecular weight of 3.8 × 105 Da. 10.4% is arabinogalactan protein with 11.4% protein and 4.5 × 106 Da molecular weight. The third part (1.2%) is glycoprotein with 47.3% protein content with 2.5×105 Da molecular weight. Gums obtained from acacia species have high molecular weight, are used as gells and thickeners, have emulsifying properties, stabilization properties and can be used for microencapsulation. It can also be used in pharmaceutical industries, has biotechnology applications and as an adhesive. Viscometry is an analytical method used to characterize polymer properties in dilute solutions. It allows fast and very simple way of determining structure, polymer concentration, polymer chain dimensions, molecular weight, and other thermodynamic properties of a polymer in solution.
Viscosity can be defined as resistance to flow, reflecting the frictional forces of all molecules (of both solute and solvent) present in solution. Capillary viscometers commonly measure the kinematic viscosity, which is the ratio of viscosity to the density of solution. To measure viscosity using a capillary viscometer, a certain amount of the polymer solution is placed in the capillary that has two marks at different levels. The flow time for the solution to pass between two lines marked in the viscometer is proportional to the kinematic viscosity of the solution. The viscosity of a solution is expressed as the sum of viscosity of the dissolved polymer and the viscosity of the solvent. Relative viscosity therefore, is defined as the ratio of solution viscosity to solvent viscosity. In order to describe an incremental change in solvent viscosity upon dissolution of a polymer, the term specific viscosity is often used. The reduced viscosity is defined as the specific viscosity divided by the concentration of the polymer.
In order to obtain the intrinsic viscosity of a polymer, the reduced viscosity data is extrapolated to zero polymer concentration. It should be noted that the units of intrinsic viscosity and reduced viscosity are "volume/mass". The intrinsic viscosity can also be treated as the natural ability of a polymer to increase the viscosity of a solution. Therefore, the intrinsic viscosity is dependent on the shape, size and molecular weight of a polymer. Dilute solution of a polymer can be defined as such a solution in which the polymer macromolecules are sufficiently far apart from one another so that their mutual interactions are eliminated and only polymer-solvent interactions take place.
The most important requirement for reliable intrinsic viscosity measurement is that the tested solution must be sufficiently dilute to eliminate polymer-polymer inter-chain interactions, so only polymer-solvent interactions and perhaps some intra-chain forces govern the size and conformation of individual polymer chains. At this state, the solution is said to be ideal-dilute, and the polymer molecule only interacts with the solvent. Therefore, the intrinsic viscosity of a polymer is only defined by the dimension of this single coil in solution. As the concentration of a polymer is gradually increased, spaces between molecules is reduced, and the molecules become more compacted producing a change in flow behavior. In diluted solutions, polymer coils must be spatially separated to prevent the formation of mechanical entanglements between the polymer chains. This process takes place at specific concentration called the critical concentration. Above this concentration, flow behavior of polymer coils is dominated by intermolecular interactions, and at concentrations below, the flow behavior is mainly due to polymer-solvent interaction. Therefore volume occupied by polymer coils is inversely proportional to the critical concentration.
Truly dilute polymer solutions are Newtonian while the presence of entanglements usually leads to non-Newtonian effects, such as time-dependent flow or visco-elasticity. Lovell argued that in the most general case the transition concentration is at to ensure truly dilute solution conditions. Industries that use gums as raw materials are in continuous search for better gums with improved physicochemical characteristics, higher quality and lower cost of production. In this work, we seek to determine the feasibility of obtaining intrinsic viscosity of pure and acetylated (modified) gum Arabic using different equations, and also compare their viscosity average molecular weights.
Arabic gum was obtain from Malam Musa Maiunguwa at kurmi Market. Ethanol, Acetic Anhydride and diethyl ether were obtained from BDH. Some of the glassware used are; beakers, pipettes, majoring cylinder, volumetric flasks. The equipment used are; clinical thermometer, Ostwald viscometer, constant temperature magnetic stirrer, and analytical weighing balance.
Sample collection
The raw exudate of Acacia Senegal gums was bought from Kurmi Market in Kano State. Malam Musa Maiunguwa assured us that the gum was from Acacia senegal which was obtained from Acacia tree in one of the bushes in Sokoto State, Nigeria.
Sample identification
The exudate sample of the gum was taken to the department of forestry, fishery and wild life in the faculty of Agricultural science at Kano University of science and technology Wudil for further identification, and was identified and certified by Malam Ibrahim Muhammad as Acacia senegal gum.
Sample purification
Impurities such as pieces of tree bark were removed by hand, and the gum was crushed to obtain smaller chunks. The gum then hydrated in distilled water for three days. The mucilage obtained was forced to drain through a calico cloth and 95% ethanol was used for precipitation of the gum. The precipitate was washed with diethyl ether, dried, powdered and stored in a desiccator for further use.
Sample modification
Acetic anhydride was used for this modification. 10 g of the gum was dissolved in 50 ml of distilled water. This makes 20% w/v concentration of the mixture. 5 g of acetic anhydride corresponding to 50% by weight of dry gum was added to the mixture. The mixture was heated at 70°C for three hours, allowed to cool, dry and was then powdered.
Preparation of gum solutions
Pure and modified gum samples were dissolved in distilled water to obtain 0.1%, 0.2%, 0.3%, 0.4% and 0.5% w/v concentrations. Solutions were gently stirred and slightly heated to obtain a uniform solution. Samples were then allowed to cool overnight.
Density measurement
Measurement of densities was carried out with the use of relative density bottles. The R.D bottle was first washed with chromic acid, rinsed with distilled water and then with alcohol, allowed to dry, and then weighted. It was then filled with distilled water and stoppered. The R.D bottle was then weighted again. Water is then removed, the bottle was washed again with alcohol and dried. It was thereafter filled with experimental liquid as before and weighted again.
Viscosity measurements
Capillary viscometers commonly measure the kinematic viscosity which is the ratio of viscosity to the density of a solution. The Ostwald viscometer was clamped to retort stand and dipped in to a 1000cm3 beaker which was filled with water. The sample solutions and reference solvent were analyzed under a temperature controlled thermostatic bath. Samples were then inserted in to the viscometer by the use of pipette until the down loop was filled. Suction pump was used to absorb the sample from the down loop to high loop where the two marks are provided. The solution was released to flow from the top mark till it reached the lower mark, and the time of flow was recorded using digital stop watch at 30°C, for each sample.
Intrinsic viscosity calculations
Viscosity of a solution containing even the slightest amount of a solute (polysaccharide), is always greater than that of a pure solvent. This is as a result of the larger size of the polysaccharide molecule as compared to that of the pure solvent.
Acetylation of gum Arabic
Acetic anhydride, acetic acid or even vinyl acetate are used in the modification of starches to produce starch acetates. During the reaction process, hydroxyl groups on the glucose units are replaced with acetyl groups to form esters. Certain factors like reactant concentration, time of reaction, catalyst and pH determine the number of acetyl groups formed in the starch matrix. In this project acetic anhydride is used instead of acetic acid. This is because acetic acid is a weak acid and its reaction with starch hydroxyl groups is reversible and the equilibrium constant does not favor the product side. The reaction is represented below (Figure 1).
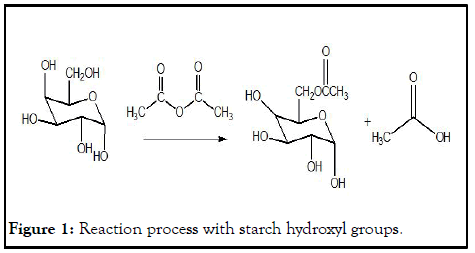
Figure 1: Reaction process with starch hydroxyl groups.
FTIR
Figures 2 and 3 shows the ftir of pure and acetylated gum arabic. The spectra of acetylated gum show the processing of new peak around 750 cm-1 to 700 cm-1 attributed to stretching indicating the presence of an ester. The peak is seen to be absent in the native gum.
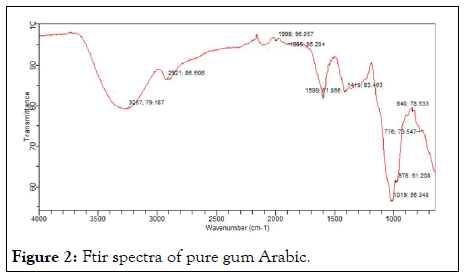
Figure 2: Ftir spectra of pure gum Arabic.
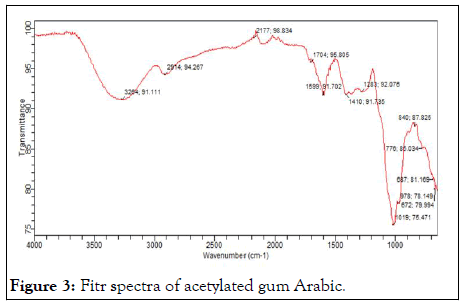
Figure 3: Fitr spectra of acetylated gum Arabic.
Densities
Figure 4 compares densities of pure and acetylated gum samples at different concentrations. The trend shows at all concentrations, modified gum sample has a higher density compared to the pure sample.
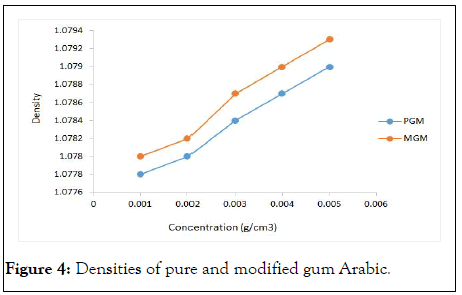
Figure 4: Densities of pure and modified gum Arabic.
Relative viscosity
The relative viscosity of pure and acetylated gum Arabic is presented in Figure 5. From the figure, it is seen that relative viscosity of acetylated gum Arabic is greater than that of pure gum Arabic. This however, does not agree with the findings of Muhamedbegovic, et al., who worked on acetylation of potato starch. In their work, they found a decrease in viscosity after acetylation of the starch. Berski, et al. made similar observation when they acetylated Oat starch. Saartrat, et al. also observed same trend after acetylation of Canna starch. The relative viscosity of acetylated starch is dependent on the uniformity of acetylation. That is whether the acetylation reached the inner lamellae of granules or just restricted to its outer part. According to Saartrat, et al., the relative viscosity of starches could either decrease or increase after acetylation. This is due to the disruption of either inter or intra molecular bonds during the acetylation process.
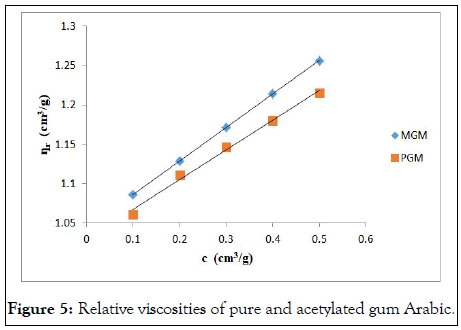
Figure 5: Relative viscosities of pure and acetylated gum Arabic.
Intrinsic viscosity
The intrinsic viscosity of pure and acetylated gum Arabic is determined using different plot methods. Table 1 shows the intrinsic viscosity of pure gum Arabic. The value differs considerably with that reported by Anderson and Rahma. This might be attributed to difference in geographic location of the parent tree, or due to difference in purification methods. However the intrinsic viscosity falls within the range reported by Renard, et al., and Yebeyen, et al.,. Taking the Huggins plot as standard, the intrinsic viscosity was determined from the plot of reduced viscosity as a function of concentration. Those plots that have Relative Errors (RE) less than 5% are considered feasible and comparable to Huggin’s method. Those methods with RE greater than 5% are considered poor and not comparable to Huggin’s method. The results in Table 1 shows Kreamer (RE 2.4%), Martin (RE 1.3%), Lyon-Toblosky (RE 2.12%), Staudinger-Heuer (RE 2.12%), Maron-Reznik (RE 3.46%) and our proposed method (RE 1.85%), are good and comparable to Huggins method.
| Method | Huggins | Kraemer | Martin | Fuoss | Fedors | Arrhenius-Rother-Hoffmann |
|---|---|---|---|---|---|---|
| η (cm3/g) | 86.43 | 83.59 | 88.28 | 80 | 106.38 | 95.033 |
| R2 | 0.812 | 0.8354 | 0.8619 | 0.7943 | 0.9807 | 0.8479 |
| RE% | - | 3.29 | 2.14 | 7.44 | 23.08 | 9.95 |
| Method | Heller | Lyon-Tobolsky | Tager | Budtov | Maron-Reznik | Kreisa |
| η (cm3/g) | 92.59 | 87.9 | 63.29 | 66.07 | 83.25 | 112.95 |
| R2 | 0.817 | 0.8606 | 0.9015 | 0.997 | 0.7745 | 0.7058 |
| RE% | 7.13 | 1.7 | 26.77 | 23.56 | 3.68 | 30.7 |
| Method | Staudinger-Heuer | Square | Square root | Mean | Proposed method | |
| η (cm3/g) | 87.89 | 75.92 | 114.06 | 45.43 | 85.45 | |
| R2 | 0.8606 | 0.5945 | 0.9075 | 0.8477 | 0.5898 | |
| RE% | 1.7 | 12.16 | 31.97 | 47.43 | 1.13 |
Table 1: Intrinsic viscosities of modified gum Arabic using different plot methods.
In Table 2, the acetylated gum Arabic shows a higher intrinsic viscosity than the native gum. The viscosity of acetylated gum is 86.43 which is higher than that of pure gum 63.40. This could be due to increase in swelling power of the acetylated gum. Acetylation also increases emulsifying ability of gums, thereby increasing the viscosity. Introduction of acetyl groups also reduces bond strength between gum molecules and thereby increasing swelling and solubility of the molecules. This enhances access of water to amorphous areas, increasing water holding capacity of the gum matrix and developing a more organised structure leading to a higher resistance to deformation and achieving a higher peak viscosity. Other methods were also used to find the intrinsic viscosity. The ones that were most comparable to Huggins and have lower RE values are; Kreamer (RE 3.29%), Martin (RE 2.14%), Lyon-Tobolski (RE 1.7%), Staudinger-Heuer (RE 1.7%), Maron and Reznik (3.68%) and our proposed method (RE 4.10%). The rest have RE values greater than 5% and are considered not comparable to Huggins.
| Method | Huggins | Kraemer | Martin | Fuoss | Fedors | Arrhenius-Rother-Hoffmann |
|---|---|---|---|---|---|---|
| η (cm3/g) | 64.59 | 63.04 | 65.43 | 100 | 68.96 | 68.23 |
| R2 | 0.9615 | 0.9635 | 0.9717 | 0.9767 | 0.9807 | 0.9815 |
| RE% | - | 2.4 | 1.3 | 54.8 | 6.76 | 5.64 |
| Method | Heller | Lyon-Tobolsky | Tager | Budtov | Maron-Reznik | Kreisa |
| η (cm3/g) | 64.9 | 65.96 | 59.17 | 53.95 | 62.35 | 74.536 |
| R2 | 0.9878 | 0.9706 | 0.8506 | 0.9984 | 0.9219 | 0.9537 |
| RE% | 0.48 | 2.12 | 8.39 | 16.47 | 3.46 | 15.4 |
| Method | Staudinger-Heuer | Square | Square root | Mean | Proposed method | |
| η (cm3/g) | 65.96 | 58.46 | 79 | 32.57 | 63.4 | |
| R2 | 0.8606 | 0.8302 | 0.9848 | 0.9775 | 0.9374 | |
| RE% | 2.12 | 9.49 | 21.8 | 49.57 | 1.84 |
Table 2: Intrinsic viscosities of pure gum Arabic using different plot methods.
It was observed that not all methods that are comparable to Huggins fitted the two samples well. Heller’s method stands out here. It was comparable to Huggins method in the pure gum sample with a relative error of 0.48%, but in the acetylated sample, it became a poor method with a RE of 7.13%. It is still not clear why this happened, but it could be due to the correlation of data between the two samples. In almost all plots used, the native gum has higher correlation than the acetylated gum. This could make some plots agreeable to Huggin’s in the native while not agreeable to Huggin’s in the acetylated sample.
The Kreiser method which this work modifies gave a very high relative error of 15.4% and 30.7% in pure and acetylated samples respectively. The method proposed herein is modification of the Kreiser method, and has fitted well and is comparable to Huggins method in both samples with RE values of 1.84% and 1.13% for pure and acetylated gum respectively (Figures 6-18).
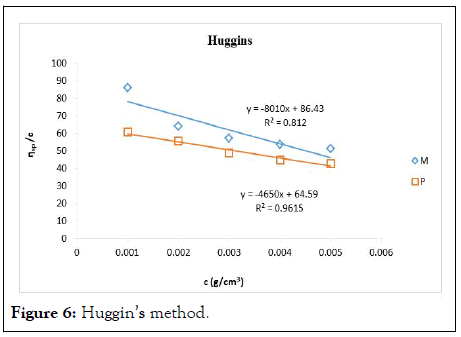
Figure 6: Huggin’s method.
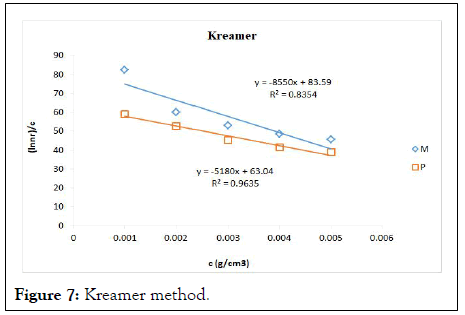
Figure 7: Kreamer method.
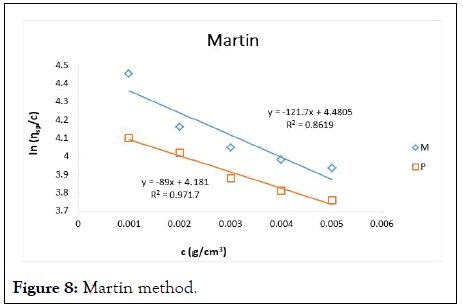
Figure 8: Martin method.
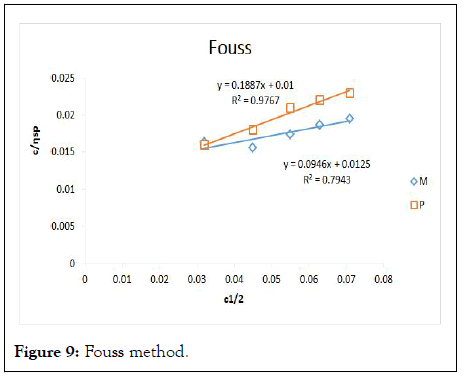
Figure 9: Fouss method.
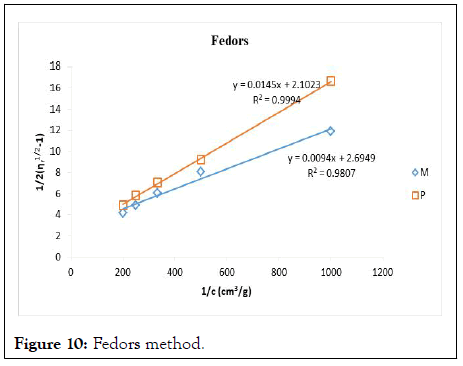
Figure 10: Fedors method.
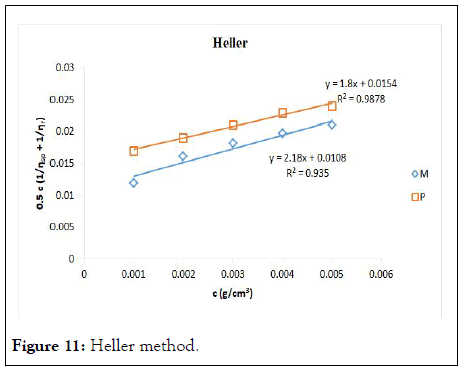
Figure 11: Heller method.
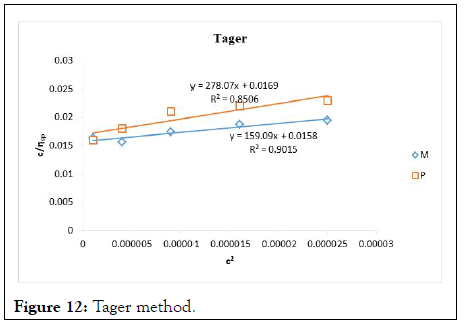
Figure 12: Tager method.
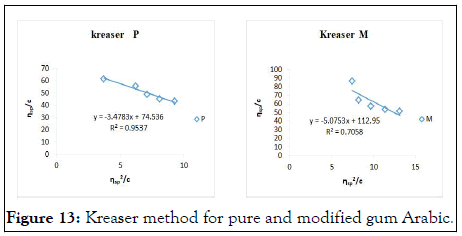
Figure 13: Kreaser method for pure and modified gum Arabic.
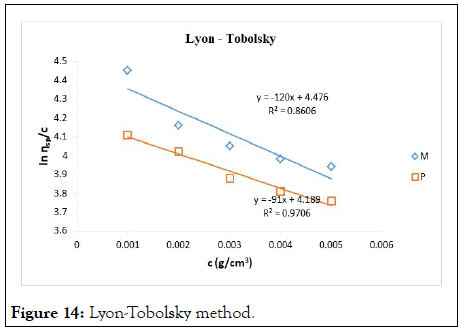
Figure 14: Lyon-Tobolsky method.
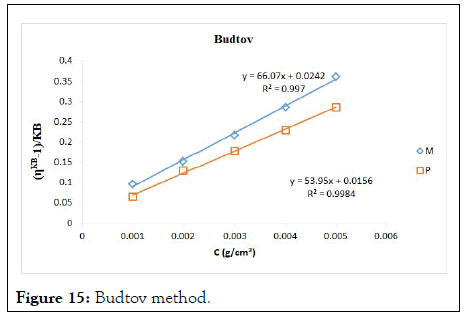
Figure 15: Budtov method.
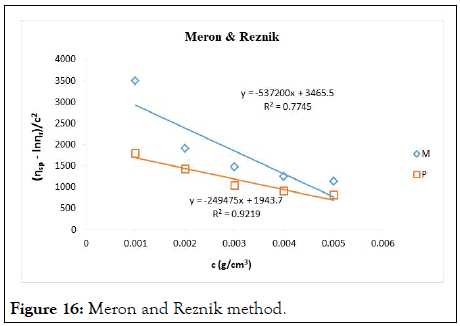
Figure 16: Meron and Reznik method.
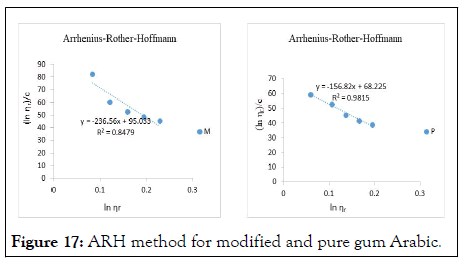
Figure 17: ARH method for modified and pure gum Arabic.
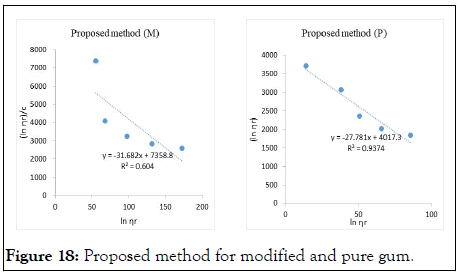
Figure 18: Proposed method for modified and pure gum.
Molecular weight determination
The molecular weight is important in determining functional characteristics of polymers such as strength and processing ability. The viscosity average molecular weight of native gum arabic found from this study is 8.64 × 106 gmol-1 which is greater than most gums such as xanthan gum (4.05 × 106 gmol-1), guar gum (1.45 × 106 gmol-1), gellan gum (1.64 × 106 gmol-1) and locust been gum (1.6 × 106 gmol-1). The molecular weight of acetylated gum Arabic was found to be 14.9 × 106 gmol-1 which is greater than that of native gum. This could be due to the substitution of hydroxyl groups with a larger ester group in the modified gum which in turn increases its solubility. Similar observation was made by Adeyanju, et al. They found that chemical modification of polysaccharides produces products with improved physicochemical and functional properties that are not available from commercial polysaccharides. They acetylated Sweitenia mycropylla gum with acetic anhydride in the presence of sodium hydroxide. The result they found showed that acetylated gum had higher values of solubility, viscosity and swelling index.
Critical concentration
The critical concentration is calculated from equation. The value for pure gum arabic was found to be 0.0155 g/cm3 and that of acetylated gum Arabic was found to be 0.01157 g/cm3. All these values are above the maximum concentration of gum samples (0.005 g/cm3). This indicates, the solutions are Newtonian and there are no entanglements between the molecules. Also it shows the viscosity determined was purely based on molecule–solvent interaction.
This work showed that the modified gum has a higher density than the pure gum, and as a result, relative viscosities and intrinsic viscosities were found to be greater in the modified gum. Also, from the different plots made, not all fitted well in calculating an accurate intrinsic viscosity for both samples. Some fitted well in the modified sample, and not that well into the pure sample. This work’s modification of the Kreiser method gave an intrinsic viscosity that is comparable to the Huggin’s method in both samples. The relative error calculated for our modified Kreiser method in far below that of the proper Kreiser method. This makes the proposed method viable. Also on comparing the molecular weights for the two samples, it was observed that the modified sample has a higher molecular weight which could be due the presence of bulkier ester groups.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
Citation: Alhassan S, Sule SY, Murtala R, Rasheed HI, Baso AA, Sheshe FA, et al. (2024) Determination of Intrinsic Viscosity of Pure and Acetylated Gum Arabic Using Different Plot Methods. J Phys Chem Biophys. 14:390.
Received: 10-Feb-2020, Manuscript No. JPCB-24-3347; Editor assigned: 13-Feb-2020, Pre QC No. JPCB-24-3347 (PQ); Reviewed: 27-Feb-2020, QC No. JPCB-24-3347; Revised: 22-May-2024, Manuscript No. JPCB-24-3347 (R); Published: 19-Jun-2024 , DOI: 10.35841/2161-0398.24.14.390
Copyright: © 2024 Alhassan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.