Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2023)Volume 14, Issue 2
Pepper (Capsicum annum L.) is an economically important vegetable and spice crops worldwide. The world pepper demand has consistently increased. However, the production of pepper has been generally low mainly due to the vulnerability of the pepper genotypes to a multitude of abiotic and biotic stresses. Propagation through seeds is restricted by short span of viability, low germination rate and requirements of extended period to produce, tissue culture provide a novel way for the asexual multiplication of pepper plants. Thus, an experiment was conducted with the main objective of this study was to determination of the combined effect of different concentration of cytokines and auxins on in-vitro shoots and root growth parameters of pepper (Capsicum annum L.) Varieties through shoot tip culture with CRD design in three replications. For shoot induction, apical shoot tips were cultured on MS medium contained 30 gm/l sucrose, 8 gm/l agar, vitamins and supplemented with three combinations (44.44 μM and 66.66 μM BAP with 2.27 μM-6.81 μM TDZ), (4.54 μM and 6.81 μM TDZ with 22.22-66.66 μM BAP) and 2.45 μM-9.8 μM of IBA combined with BAP (22.22 μM-88.88 μM) and TDZ (2.27 μM-9.08 μM) were examined. In the tested cytokinins combinations, 4.54 μM TDZ and 6.81 μM TDZ with 66.66 μM BAP for Bako local and 44.44 μM BAP with 4.54 μM TDZ and 6.81 μM TDZ for Marko fana were resulted best performance in shoot growth parameters. For the tested cytokinins to auxin combinations, 4.54 μM-6.81 μM TDZ+7.35 μM IBA for Bako local and 6.81 μM TDZ+4.9 μM IBA for Marko fana variety showed best shoot growth performance. In root induction, MS media fortified with 2.45 μM-9.8 μM IBA and 2.65 μM-10.6 μM of NAA and two combinations 7.35 μM IBA with 2.65 μM-7.95 μM NAA and 5.3 μM NAA with 2.45 μM-7.35 μM of IBA for Marko fana variety and 4.9 μM of IBA with 2.65 μM-7.95 μM NAA and 2.65 μM NAA with 2.45 μM-7.35 μM IBA for Bako local variety were examined. Among auxin combinations, 2.45 μM and 4.9 μM of IBA with 2.65 μM of NAA for Bako local and 4.9 μM of IBA with 5.3 μM of NAA and 7.35 μM of IBA with 2.65 μM of NAA for Marko fana variety were resulted best performance. Under DAS-ELISA test, three pepper viruses were detected and they were eliminated by apical shoot tip tissue culture method. In the acclimatization, the effects of PGRs and four different culture substrates on the survival of in vitro propagated pepper plantlets were examined. This result showed 81.11% and 70.10% plantlet survival for Marko fana and Bako local pepper varieties, respectively. In conclusion, it is beneficial to use the in vitro propagation protocols developed in this study for mass micro propagation and virus elimination to overcome the challenges of conventional cultivation of pepper. Further studies must focus on the performance of the in vitro propagated pepper plantlets for growth characters, yield/yield related parameters and yield quality parameters in field establishment in order to arrive at a sound conclusion.
MS-medium; Auxin; Cytokinins; Marko fana; Bako local; Growth regulators, DAS-ELISA
Pepper (Capsicum) is a new world genus of the Solanaceae family originated and domesticated in the American tropics. Capsicum is derived from the Greek word “Kapsimo” meaning to bite. All species in the genus are still not known, but there are thought to be 25 species-30 species of Capsicum, of which five namely C. annuum, C. baccatum, C. chinense, C. frutescens, and C. pubescens have been domesticated and currently cultivated. In many countries of the world, pepper is a cash crop with high domestic and export value [1-3]. Chilli industries have grown from regional food supply for tourists to an international industry, competing on the global market. The nutritive value of Capsicum is high and it is an excellent source of vitamins C (ascorbic acid), A, B-complex and E vitamins along with minerals like molybdenum, manganese, folate, potassium, phosphrus, calcium and thiamine. Chilli contains seven times more vitamin C than orange. Beta-carotenoids, vitamins C and A in chilies are powerful antioxidants that destroy free radicals. At present there is an expanding pepper market for dietary consumption. The oleoresin from Paprika is currently used in a wide assortment of foods, drugs, cosmetics as well as for improving the feather color of flamingos in zoos or koi fishes in aquarium. The pharmaceutical industry uses capsaicin as a counter irritant balm for external application and to alleviate pain [4,5]. Because of their unique fruit shapes and bright fruit color, several pepper species have been widely used as ornamentals. The crop is exported as dried ripe fruits or as oleoresin extracted from the fruit. In addition, it also serves as an income generating crop for small-scale farmers [6].
In Ethiopia the very common type of capsicum is hot pepper. Traditionally Ethiopians distinguish three kinds of hot peppers “berbere” red matured pungent fruit,”karya” the immature green fruit and “mitmita” the small very pungent fruits. The powder from dried ripe fruit of hot pepper is used as spice to flavor “wot” an Ethiopian stew in daily traditional meal [7,8]. The fully matured green pods are eaten as salads. Meals without pepper are conceded as tasteless to many Ethiopians.
Production of pepper is scattered throughout the country. The pepper production area for both green pod and dry pod in the country is estimated to be about 54082 hectares in 2007 hectares and 64774 hectares in 2008 with the production of 110095 tons and 116739 tons in the respective years [9]. The yield decreased from 10.7 tons to 9.2 tons per hectare due to viral disease and poor management practices. Nowadays, smallscale farmers produce the largest portion of hot pepper in the country. It is widely cultivated in Amhara and Oromyia regions. Red pepper and chilli are the leading vegetable and spices grown in the country [10]. The central (Eastern and Southern Shoa), western, North western (Wollega, Gojjam) and the southern part of the country are the potential pepper producing areas. Currently most of the produce comes from Alaba, Meskanina, Marko and Siltie zones. The production of pepper in Ethiopia has been generally low over the last several years. The decline of hot pepper production in the country is mainly attributed to poor varieties, poor cultural practices, the prevalence of fungal (blights) and bacterial as well as viral diseases.
Vulnerability of the pepper genotypes to a multitude of abiotic and biotic stresses has restricted their potential yield. Abiotic factors that significantly diminish the yield and quality of peppers include extreme temperature, moisture, light, nutrients and pH. Biotic factors include susceptibility of pepper to various fungi, bacteria and viruses. The phyto pathological problem that poses a great threat to cultivated pepper is viral infection. Peppers are prone to a wide variety of viruses including tobacco mosaic virus, tobacco etch virus, cucumber mosaic virus, potato virus Y, pepper mottle virus, ring spot virus, potyvirus and tospovirus but the most prevalent pepper viruses in Ethiopia are Ethiopian pepper mottle virus and potato virus Y. Many researchers showed that yield loss by viral diseases in most surveyed areas of Ethiopia was estimated at 15%-50%. More than 90% viral disease incidences and complete crop failure have been reported from some places in Ethiopia. Particularly, severe yield loses may occur when the crop is infected with two or more viruses synergistically and at early growth stages. The relative importance of viruses on pepper is quite variable across regions, where some viruses are endemic to a particular region [11-13].
Ethiopian Pepper Mottle Virus (EPMV), a potyvirus occurring in mixed or single infection, is the most important virus in the rift valley and Southern parts of Ethiopia. Frequent and severe outbreak of viral disease in hot pepper and absence of even one single study in the production of virus free pepper plantlets in the country have prompted this study. Pepper constantly shows severe virus symptoms throughout Ethiopia and in the rift valley belt in particular. Farmers in general, state farms in the past and recently private investors have often banned the production of the crop because of unacceptably high losses due to crop viral infection. Thus, yield and production areas of pepper remained constantly low in Ethiopia over the last few years. It is impossible to cure virus infected plants. However, biotechnology techniques involving plant tissue culture and recombinant DNA technologies are powerful tools that can complement conventional breeding and promote Capsicum improvement. To alleviate the great economic loss of pepper production by virus infection and to produce commercially high quality of pepper and pepper products, production of virus free pepper plants play crucial role. One way of pathogen elimination technique is apical shoot tip tissue culture. On this regard the present study concentrates on the production of virus free pepper plants from virus infected peppers through shoot tip tissue culture would help to solve the problems [14-16].
Thus the main objective of this study was to determination of the combined effect of different concentration of cytokines and auxins on in-vitro shoots and root growth parameters of pepper (Capsicum annum L.) Varieties through shoot tip culture. More specifically:
• To determine the combined optimum concentration of cytokines (BAP and TDZ) for shoot growth parameters of Marko Fana and Bako local pepper varieties.
• To determine the combined optimum concentration of auxins (IBA and NAA) for root growth parameters of Marko Fana and Bako local pepper varieties.
• To acclimatize plantlets and determine the survival rate of plantlets for field production of Marko Fana and Bako local pepper varieties.
The experimental laboratories sites
The experiment was conducted in the Tissue Culture Laboratory of Melkassa Agricultural Research Center (MARC) which is located 124 km East of Addis Ababa at 80 24’ N latitude and 390 21’ E longitudes and at elevation of 1550 m.a.s.l. It is characterized by low and erratic rainfall with unimodal pattern of precipitation [17]. The maximum and minimum annual mean temperature is 33℃ and 10.8℃, respectively. The virus indexing experiment was conducted in the Virology Laboratory of Ambo Agricultural Research Center (AARC) which is located 125 km west of Addis Ababa at 8°59′N latitude and 37°51′E longitude with an elevation of 2101 m.a.s.l.
Experimental plant materials
The seeds of two released commercial varieties of pepper namely Marko fana and Bako local were used. The variety Marko fana was obtained from Melkassa Agricultural Research Center (MARC) and it is highly susceptible to many pathogens such as viruses, fungi and bacteria whereas the variety Bako local was obtained from Bako Agricultural Research Center (BARC) and it is less susceptible to pathogens.
Treatments and experimental design
The experiment of the present study was laid out in a Completely Randomized Design (CRD) in factorial arrangement with three replications of each treatment and each replicate consisted of three explants per jar. Four series of experimental stages (shoot induction and elongation, root induction, virus indexing and acclimatization) were examined to develop an efficient and reliable in vitro propagation protocol for the two commercial varieties of pepper (Marko fana and Bako local). In the shoot regeneration and multiplication stage, two types of cytokinins with five different levels of concentrations were used. These cytokinins were Benzyl Amino Purine (BAP) with the concentration of 22.22 μM, 44.44 μM, 66.66 μM and 88.88 μM and Thidiazuron (TDZ) with the concentration of 2.27 μM, 4.54 μM, 6.81 μM and 9.08 μM. The combinations of BAP and TDZ as well as with IBA were also tested. These combinations were 66.66 μM of BAP+4.54 μM of TDZ and 4.54 μM of BAP+66.66 μM of TDZ and each concentration of BAP and TDZ combined with IBA. MS medium without any cytokinins was included as a control [18].
In the root induction stage, two types of synthetic auxins each with four levels of concentrations were tested on this experiment. These auxins were Naphthalene Acetic Acid (NAA) with the concentration of 2.65 μM, 5.3 μM, 7.95 μM and 10.6 μM and Indole-3-Butyric Acid (IBA) with the concentration of 2.45 μM, 4.9 μM, 7.35 μM and 9.8 μM. Two combinations of these rooting hormones (7.35 μM IBA+5.3 μM NAA and 7.35 μM NAA+5.3 μM IBA) were examined. MS medium without auxin was also incorporated as control treatment. In this phase all the treatments were equally assigned to a full strength MSmedium [19]. In the third phase of the experiment (acclimatization phase) the effect of PGR and the culture substrates were used as treatments to examine the survival of the in vitro regenerated plantlets. The major culture substrates used on this experimental phase were sand alone, soil alone, compost alone and the three mixtures in the ratio of 2:1:1, respectively on both pepper varieties. In the fourth phase of the experiment, virus indexing stage, the in vitro regenerated pepper plantlets were tested via DAS-ELISA serological test in order to confirm the presence or absence of viruses as well as the effectiveness of shoot tip tissue culture in the elimination of viruses from virus infected Marko fana and Bako local pepper varieties.
Preparation of stock solution and media
MS media stock solution: MS basal media is the most widely used and recommended basal medium for micro propagation of most plant species and was selected for this experiment. To carry out this experiment, macronutrient and micronutrient stock solutions, vitamin stock solutions and plant growth regulator stock solutions were prepared. In all cases, the stock solutions were prepared by weighing the recommended amounts of the chemicals using an electronic sensitive balance, and were dissolved in sterilized distilled water. Finally, the stock solutions were poured into labeled plastic bottles and stored into a refrigerator at the temperature of 4℃. The components of each solution were described below. The components for the preparation of macro nutrients stock solutions consisted of NH4NO3, KNO3, CaCl2.2H2O, MgSO4.7H2O and KH2PO4, while the preparation of micro nutrients mainly include H3BO3, MnSO4.4H2O, ZnSO4.7H2O, KI, FeSO4.7H2O, Na2Fe-EDTA, Na2MoO4.2H2O, CuSO4.5H2O and CoCl2.6H2O. Vitamins are organic compounds that are well functional in trace amount in the given medium to enhance the growth of plants in vitro. Since they are required in small amount, they are prepared in solutions form. The major vitamins used in this experiment to prepare vitamin stock solutions Myo-instol, Glycine (Glycocoll), Nicotinic acid, Pyridoxine-HCl (B6), and Thiamine-HCl (B1).
Plant growth regulators stock solutions: Four Plant Growth Regulators (PGRs) with different concentration were used for this experiment including N-6-Benzyl Amino Purine (BAP), Thidiazuron (TDZ), α-Naphthalene Acetic Acid (NAA) and 3- Indole Butric Acid (IBA). All PGR stock solutions were prepared by weighing the required amount of the chemical using an electronic sensitive balance and dissolving them in sterilized distilled water. To enhance the dissolution, three drops of 1N NaOH and 1N HCl were consistently used for auxins and cytokinins, respectively. Thereafter, each stock solution was adjusted to the desired volume in a volumetric flask using sterilized distilled water. Finally, the PGR stock solutions were poured into properly labeled media bottles and stored in a refrigerator at the temperature of 4℃.
Composition and preparation of nutrient media: In all cases, the culture media were prepared by measuring the recommended amounts of MS stock solutions supplemented with 3% (w/v) sucrose as a carbon source and 0.8% (w/v) agar as solidifying agent. After mixing up all media components together with the combined PGR and adjusting the volume, the pH of the culture medium was adjusted to 5.8 with either 0.1%N HCl or 0.1% N NaOH. Then, solidifying agent (agar) was added into the medium. Heat stable MS media was autoclaved at 15 pounds per square inch (psi) at 121℃ for 20 minutes. However the heatlabile PGRs and some amino-vitamins were filtered through a bacteria-proof membrane (0.22 μm) filter and added to the autoclaved medium after it has cooled enough. Then autoclaved MS media was allowed to cool in sterile environment after which it was ready for use. After autoclaving, 40 ml of the respective medium was poured into culture vessels retained in the transfer room for a maximum of three days prior to use for shoot initiation and root induction.
Explants sterilization and explant preparation
Explant sterilization procedures were carried out on laminar airflow cabinet bench. Glasshouse grown shoots were cut and put into tap water in 50 ml glass bottle and rinsed three times. Explants were washed with largo soap and rinsed three times with tap water. The explant then immersed in 3% coccid dissolved in 1000 ml sterilized distilled water for 30 minutes and then the explants were rinsed three times with sterilized distilled water followed by immersing the explant in to 150 ml/l of 5% chlorine containing berekina and added 3 drops-4 drops of Tween-20 for 20 minutes to facilitate wetting of the explants. Finally the explants were rinsed three times with sterilized distilled water. About 1 cm shoot tips were excised from each explant and cultured on the prepared MS basal media alone or supplemented with different concentration of PGRs.
Experiment I: The combined effect of cytokinins on in vitro shoot induction and multiplication
Pepper apical shoot-tip (0.5 cm) explants were derived from three week-old glasshouse grown seedlings and inoculated on a shoot bud induction medium consisting of MS basal medium supplemented with (0, 22.22, 44.44, 66.66 and 88.88) μM of Benzyl Amino-Purine (BAP) and (0, 2.27, 4.54, 6.81 and 9.08) μM of Thidiazuron (TDZ) alone or in combinations with 2.45 μM-9.80 μM Indole-3-Butric Acid (IBA). The cultures were maintained in a growth room at a temperature of 25℃ ± 2℃and 16-h photoperiod provided by white fluorescent tubes. Then all pepper shoot growth parameters were recorded after three to four weeks of culture with monthly interval.
Experiment II: The combined effect of auxins on in vitro root induction and multiplication
The elongated shoot buds (about 2 cm long) obtained from shoot-tip explants were excised and cultured in rooting media consisting of MS medium treated with (0 μM, 2.45 μM, 4.9 μM, 7.35 μM and 9.8 μM) of Indole-3-Butyric Aid (IBA) and (0, 2.65, 5.3, 7.95 and 10.6) μM α-Naphthalene Acetic Acid (NAA) with their combinations for the rooting of shoot buds. Then the cultures were maintained in a growth chamber at a temperature of 25℃ ± 2℃ and 16-h photo period provided by white fluorescent tubes. Then the root growth parameters were recorded at monthly interval.
Experiment III: Virus indexing techniques
In order to confirm the presence or absence of viruses for in vitro propagated plantlets of peppers from virus infected Marko fana and Bako local pepper varieties, serological tests were conducted. In this study due to the shortage of ELISA kit, only polyclonal antibodies specific to potato virus y (PVY), Ethiopian Pepper Mottle Virus (EPMV) and Pepper Venial Motile Virus (PVMV) were used to test the occurrence of these viruses in the given pepper variety samples. In the two pepper varieties used in this study, both the mother plant before the experiment and the in vitro regenerated plantlets after the experiment were tested by the Double Antibody Sandwich Enzyme Linked Immunosorbent Assay (DAS-ELISA). A total of 120 samples for the two commercially released pepper varieties (60 sample for Marko fana and 60 samples for Bako local variety) were tested and the positive results were used as a mother plant for this experiment in order to confirm the efficiency of shoot tip tissue culture in virus elimination.
This test was carried out using a polyclonal antibody specific for each virus, ELISA microrplates and different buffers important for DAS-ELISA. It involves the addition of a specific antibody to the test plate where it adsorbs, followed by addition of the test samples. Specific particles in the sample are immobilized on the antibody film. Subsequently, enzyme labeled antibody is added and becomes immobilized on the sample particles. Test particles were then quantified by the addition of enzyme substrate, through the colorimetric or fluorometric detection of the reaction product. Buffers required in DAS-ELISA kit and their preparation procedures:
• In a clean flask, mixed 2 ml of buffer 1(dispensed in a bottle) with 8 ml of distilled water for each plate.
• Dissolved each packet of buffer 2A (PBS) in 1,000 ml of distilled water and mixed thoroughly with magnetic stirrer.
• To one liter of buffer 2A (PBS), added 20 drops (0.5) of buffer 2B (Tween-20) and mixed well with pasture pipette.
• Dissolved the contents of one packet of buffer 3 with a very small amount (approximately 10 ml) of PBS-T until it is completely dissolved and then increased the volume of the solution with buffer PBS-T up to 200 ml.
• Dissolved the contents of one packet of buffer 4 with a very small amount (approximately 5 ml) of PBS-T until it is completely dissolved and then increased the volume of the solution with buffer PBS-T up to 20 ml.
• Mixed 2 ml of buffer 5 ml with 8 ml of distilled water for one plate.
For each plate, 35 μl of antibody (IgG) of the virus to be detected was mixed with 10 ml of coating buffer ( the mixture called coating solution) and mixed well by avoiding formation of foam in the solution and then 100 μl of the coating solution was added to each wells in the ELISA plates. The plates were covered with a piece of masking tape and incubated at 37℃ for 3 hours-4 hours. After the incubation period, plates were emptied and drain immediately on adsorbent paper and each wells of the micorplates were washed three times with PBS-T by soaking for 3 minutes and drain.
In this experiment, 1 gm of leaves taken from each samples was extracted by grinding and blending in 4 ml extraction buffer then added 100 μl of the grinded samples sap in to the wells of the micorplates and sealed the plate with a piece of masking tape or Para film and incubated it in a standard refrigerator (4℃) overnight. After refrigeration period, for each plate, 35 μl of each conjugate antiserum (IgG-AP) were mixed with 10 ml of conjugate buffer (the mixture called conjugate solution) and added 90 μl of the conjugate solution to each well of the plate.
Then the plates were sealed and incubated at 37℃ for 3 hours-4 hours or at room temperature (± 25℃) for 5 hours. After incubation period, each well of the micorplates were washed three times with PBS-T by soaking for 3 minutes and then drain.
To observe the development of the reaction one substrate tablet was dissolved in 10 ml of substrate buffer to form substrate solution and 80 μl of the substrate solution was added to each well of the plate. Then the reaction occurred when the plates were left for 30 minutes to 60 minutes at room temperature. In the presence of the substrate for the enzyme (alkaline phosphotransferase) conjugated with the secondary antibody, catalyzed reaction signals visualization of yellow color for the virus positive samples. However, the healthy control and buffer wells remain clear.
Experiment IV: Acclimatization
The well rooted in vitro propagated pepper shootlets from each PGR treatments were gently removed from the culture media flasks and the roots were washed in tap water to remove traces of agar that prevent the absorption of nutrients from the acclimatization culture substrates by roots. The plantlets were planted into four different culture substrates (sand alone, sterile soil alone, compost alone and their mixture in the ratio of 2:1:1) containing pots and then covered by plastic bags (in order to keep humidity) for one to two weeks in glasshouse by irrigating water daily. From this experiment, the effects of PGRs and culture substrates on the survival of the in vitro propagated pepper plantlets were examined by calculating the number of survived shootlets and died shootlets among the total transferred plantlets.
Data recording
All the responses for every treatment were recorded for every shoot tip culture initiated. For both varieties of shoot tip cultures initiated on each treatments, number of nodes produced/explants, number of leaves, length of shoot and number of shoots/explant, number of roots and root length, survival capacity and presence or absence of viruses were counted and recorded. These response data were used to choose the type and optimum concentration of growth regulators used in the media giving the maximum morphological characteristics of shoots and roots for each variety subjected to a particular treatment.
Number of days to shoot and root emergence: Number of days to shoot and root emergence is the length of time needed by the explant to produce shoots from the first day of culturing in the shoot initiation and root induction media respectively.
Mean shoot number: This parameter referred to the average number of dissectible shoots regenerated from each cultured explants. In order to estimate the multiplication rate of pepper in vitro, the number of shoots was counted three times i.e., at each sub-culturing cycle of the shoot multiplication medium. During the three successive cycles of shootlets proliferation the cumulative number of the formed shootlets per explant was counted at monthly intervals.
Bud number: This parameter referred to the average number of buds developed from each cultured explant. In order to determine the response of pepper in in-vitro, the number of buds developed on each explants was counted three times after four weeks of culture on shoot multiplication medium (at monthly intervals).
Leaf number: counting the average number of leaves developed from each cultured shoot explant. In order to determine the performance of pepper in in-vitro, the number of leaves developed on each explants was counted three times after four weeks of culture on shoot multiplication medium at monthly interval.
Length of shoot and root: This is the average length of shoots and roots developed from each explant. The parameter was measured using an autoclaved square paper and by a well sterilized ruler (measuring tape). The data for the length of shoot was recorded three times after four subsequent week’s culture in the shoot multiplication medium and root length was only taken once after two weeks of incubation.
Root number: This parameter also referred to the average number of roots regenerated per shootlets explant from each cultured explants. In order to estimate the multiplication rate of pepper in vitro, the number of main roots was counted after four weeks of growing in root induction media.
Shoot and root fresh weight: This is the average fresh weight of regenerated shoots and roots on each explant. Shoot fresh weight and root fresh weight were weighed at the end of the propagation phases in both the shoot and root induction medium, using an electronic sensitive balance.
Shoot and root dry weight: This parameter referred to the average dry weight of regenerated shoots and roots on each explants after oven drying at 60℃. Similarly the shoot dry weight and root dry weight was weighed at the end of the shoot multiplication and root induction phases.
Survival rate: Is the competence or the ability of the in vitro derived plantlets to ensure in the ex-vivo condition after one week of acclimatization in the greenhouse.
Presence or absence of viruses: The in vitro propagated pepper plantlets were checked whether they are positive or negative from pepper infecting viruses using DAS-ELISA methods before mass propagation.
Data analysis
The experimental design was simple Complete Randomized Design. All experiments were carried out in three replications. Data were subjected to Analysis of Variance (ANOVA) and significant differences among treatments were determined by Duncul’s Multiple Range Test (DMRT) using the SAS (version 9.2) software package for the differentiation of the effect of treatment and genotype on shoot and root induction. Percent of shoots freed from viruses by shoot tip culture was calculated. For all the data analysis, probability level of 5% (p ≤ 0.05) was considered for statistical significance.
The combined effect of different concentration of BAP and TDZ on shoot growth parameters of marko fana and bako local pepper varieties
As indicated in Tables 1 and 2, the number of shoots per explant showed significant difference among treatment combinations of BAP and TDZ compared with the MS media containing BAP or TDZ alone. Anilkumar and Nair, reported the effectiveness of combination of 31 μM BAP and 4.6 μM kinetin for multiple shoot induction from shoot-tip explants in Capsicum annuum L. CV. early California wonder. However, in the present study among the four combinations of BAP and TDZ, 44.44 μM of BAP combined with 4.54 μM of TDZ was produced significantly maximum mean number of shoots (21.51) which showed two fold increased than that of MS media having 44.44 μM of BAP alone (10.95) followed by 22.22 μM of BAP combined with 6.81 μM of TDZ (19.75) for the variety Marko fana. When 66.66 μM of BAP combined with 2.27 μM-6.81 μM of TDZ, only 66.66 μM of BAP combined with 4.54 μM of TDZ produced significantly maximum mean number of shoots (18.33) per explant. Sanatombi and Sharma reported that very high levels of BAP (66.6 μM-88.8 μM) and kinetin (92.9 μM-116.2 μM) were necessary for maximal shoot proliferation from shoot-tip explants of Capsicum frutescens L. cv. ‘Uchithi’ by producing a maximum of 5.6 shoots per explant on MS medium containing 22.2 μM BAP in combination with 4.6 μM kinetin. Whereas on the variety Bako local explants cultured on full MS media supplemented with 44.44 μM of BAP combined with 2.27 and 4.54 μM of TDZ, maximum mean number of shoots per explant (18.47) and (18.34) were produced, respectively. However, when 6.81 μM of TDZ was combined with the four levels of BAP, none of the combinations produced better number of shoots per explant as compared with 6.81 μM of TDZ alone.
In case of shoot length, the variety Marko fana explants cultured on MS medium containing 44.44 μM of BAP alone was observed to have the longest mean shoot length (4.35 cm) followed by the combination of 44.44 μM of BAP with 4.54 μM of TDZ (3.93 cm) than other treatment combinations. Therefore, 44.44 μM of BAP alone showed that it is applicable for the shoot length. When 66.66 μM of BAP combined with (2.27, 4.54 and 6.81) μM of TDZ, only 66.66 μM of BAP combined with 4.54 μM of TDZ produced significantly longer shoot length as compared to 66.66 μM of BAP alone and other combinations. On the other hand, on the Bako local pepper variety significantly longer shoot (3.65) was recorded on explants cultured on full strength MS media supplemented with 44.44 μM of BAP combined with 2.27 μM of TDZ as compared to individual treatments of these two PGRs and other combinations. Therefore this combination is applicable and optimum for shoot length of this variety. Generally, as the concentration of TDZ increases, making BAP constant and vice versa the length of the shoot gradually decreased for both pepper varieties. The possible justification for this is that as the concentration of these cytokinins increased beyond the optimal need of the plant, they inhibit the release of endogenous cytokinins and the assimilation of the given nutrients by inhibiting the activities of enzymes.
Concerning mean number of buds differentiation per explant, the results presented in Tables 1 and 2 indicated that the combination of TDZ with BAP showed significant differences among treatments and varieties. Previous report by Sanatombi and Sharma, revealed that axillary shoot-tip explants produced multiple shoot buds when cultured on MS medium containing 8.8 μM-44.4 μM of BAP combined with 4.6 mM kinetin. But in the present study for the variety Marko fana, when 44.44 and 66.66 μM of BAP combined with 2.27, 4.54 and 6.81 μM of TDZ, significantly maximum mean number of buds (24.51) was obtained at the combination of 44.44 μM of BAP with 4.54 μM of TDZ followed by 66.66 μM of BAP with 4.54 μM of TDZ (23.81) compared with MS media containing 44.44 μM of BAP (18.33) alone and 66.66 μM of BAP alone (22.54). However, when 6.81 μM of TDZ combined with 22.22 μM, 44.44 μM and 66.66 μM of BAP, none of the combinations produced better number of buds than 6.81 μM of TDZ alone (23.62). On the other hand, the variety Bako local produced significant mean number of buds as MS media supplemented with 66.66 μM of BAP combined with 4.54 and 6.81 μM of TDZ (23.81) and (22.43), respectively. However, when 44.44 μM of BAP combined with the above three levels of TDZ, none of these combinations produced significant number of buds compared with MS media treated only 44.44 μM of BAP (20.75).
Among the organs of plant parts, leaves were the critical part for the survival of the plant by photosynthesizing food as well as in water balance: The result data indicated that there was significant difference between varieties and treatments on the production of leaves. This result showed that when 44.44 μM of BAP was combined with (2.27 μM-6.81 μM) of TDZ, in the variety Marko fana, significantly maximum mean number of leaves (25.75) were found in explants cultured on full strength MS media supplemented with 44.44 μM of BAP combined with 4.54 μM of TDZ compared with other hormonal combinations. However, when 66.66 μM of BAP was combined with the above three levels of TDZ, none of these combinations produced significant change in mean number of leaves compared to 66.66 μM of BAP alone. On the variety Bako local, significantly maximum mean number of leaves per explant were found in explants cultured on MS media supplemented with 66.66 μM of BAP combined with 6.81 μM of TDZ (24.44 leaves).
In this study the combination of TDZ with BAP revealed significant variations on mean shoot fresh and dry weight among the different cytokinins combinations and between varieties at (P<0.05). In the variety Marko fana, significant mean shoot fresh weight (0.77 g) and shoot dry weight (0.57 g) per explant was recorded in explants cultured on full strength MS medium fortified with 4.54 μM of TDZ plus 44.44 μM of BAP which is the best and optimum combination for this variety. When the explants were cultured on MS media supplemented with 6.81 μM of TDZ alone a maximum shoot fresh weight (0.75 g) and shoot dry weight (0.52 g) were obtained. When the explants were cultured on MS media fortified with 66.66 μM of BAP alone, (0.66 g) of shoot fresh and (0.35 g) of shoot dry weight were recorded. On the Bako local pepper variety, when 6.81 μM of TDZ combined with (22.-66.66) μM of BAP, significant mean shoot fresh weight (0.74 g) and dry weight (0.52 g) per explant were obtained at the combination of 6.81 μM of TDZ with 66.66 μM of BAP.
| Cytokinins concentration in (µM) | No. ofdays for shoot emergence(n) | Mean No. of shoots/expl (n) | Meanshoot length/exp (cm) | Mean No. of buds/exp (n) | Mean No. of leaves/ exp(n) | Shoot fresh Wt/expl (g) | Shoot dry Wt/expl (g) | |
|---|---|---|---|---|---|---|---|---|
| BAP | TDZ | |||||||
| 44.44 | 0 | 11.75c | 10.95d | 4.35a | 18.33e | 14.66ef | 0.49c | 0.26g |
| 44.44 | 2.27 | 11.36cde | 12.22cd | 3.11f | 19.02cd | 15.32e | 0.50c | 0.31ef |
| 44.44 | 4.54 | 10.25d | 21.51a | 3.92ab | 24.51a | 25.75a | 0.77a | 0.57a |
| 44.44 | 6.81 | 12.32b | 13.33c | 3.51c | 22.33b | 21.63c | 0.55b | 0.34cde |
| 66.66 | 0 | 13.67ab | 6.53f | 3.22ef | 22.54b | 23.52b | 0.66ab | 0.35cd |
| 66.66 | 2.27 | 13.52ab | 6.51f | 3.68b | 22.33b | 17.41d | 0.53bc | 0.36c |
| 66.66 | 4.54 | 11.37cde | 18.33b | 3.81ab | 23.81ab | 23.43b | 0.58ab | 0.41bc |
| 66.66 | 6.81 | 13.74a | 8.51e | 3.43cd | 20.33c | 22.33bc | 0.45d | 0.28f |
| 0 | 4.54 | 11.56cd | 17.30b | 3.75b | 17.67ef | 13.58ef | 0.51bc | 0.29ef |
| 22.22 | 4.54 | 11.45cde | 16.72bc | 3.34cd | 19.75cd | 18.33d | 0.53bc | 0.31e |
| 44.44 | 4.54 | 10.25d | 21.51a | 3.92ab | 24.51a | 25.75a | 0.77a | 0.47b |
| 66.66 | 4.54 | 11.37cde | 18.33b | 3.81ab | 23.81ab | 23.43b | 0.58ab | 0.38bc |
| 0 | 6.81 | 11.25cdef | 19.53ab | 3.01fg | 23.62ab | 25.62a | 0.75a | 0.52ab |
| 22.22 | 6.81 | 12.27b | 19.75ab | 3.33cd | 20.51c | 17.05d | 0.46d | 0.31ef |
| 44.44 | 6.81 | 12.32b | 13.33c | 3.51c | 22.33b | 21.63c | 0.55b | 0.32e |
| 66.66 | 6.81 | 13.74a | 11.91d | 3.43cd | 20.33c | 22.33bc | 0.45d | 0.28f |
| Mean | 12.01 | 14.77 | 3.57 | 21.61 | 20.74 | 0.57 | 0.36 | |
| CV | 9.23 | 6.33 | 7.25 | 11.15 | 10.02 | 3.55 | 2.92 | |
Table 1: Effect of different concentration of BAP and TDZ combinations on morphogenetic response of shoot growth parameters of Marko fana cultured on full strength MS medium using shoot tip as explant.
| Cytokinins in µM | No. of daysfor shoot emergence(n) | Mean No. of shoots/expl (n) | Mean shoot length/exp (cm) | Mean No. of buds/exp (n) | Mean No. of leaves/exp(n) | Shoot fresh Wt/expl (g) | Shoot dry Wt/expl (g) | |
|---|---|---|---|---|---|---|---|---|
| BAP | TDZ | |||||||
| 44.44 | 0 | 12.52de | 8.66g | 3.52b | 20.75c | 19.65e | 0.56cd | 0.33c |
| 44.44 | 2.27 | 12.45def | 18.47a | 3.31c | 17.02e | 17.32ef | 0.51d | 0.25ef |
| 44.44 | 4.54 | 10.92e | 14.34b | 3.52b | 23.53ab | 20.34dc | 0.66b | 0.37b |
| 44.44 | 6.81 | 14.27bc | 13.32de | 3.55a | 21.38c | 21.63c | 0.57c | 0.32c |
| 66.66 | 0 | 13.75c | 12.5e | 2.97d | 19.64d | 16.66f | 0.51d | 0.25ef |
| 66.66 | 2.27 | 12.59d | 6.95h | 2.68e | 22.33bc | 18.51e | 0.56cd | 0.26e |
| 66.66 | 4.54 | 14.33bc | 10.43f | 3.21c | 23.81a | 20.63d | 0.57c | 0.28d |
| 66.66 | 6.81 | 15.66a | 7.61gh | 3.11cd | 22.43b | 24.44a | 0.72a | 0.35bc |
| 0 | 4.54 | 12.33ef | 16.75ab | 3.37bc | 21.54b | 22.57b | 0.72a | 0.45a |
| 22.22 | 4.54 | 12.45d | 17.11ab | 3.44bc | 22.75ab | 22.23bc | 0.64bc | 0.25ef |
| 44.44 | 4.54 | 10.92e | 18.34a | 3.52b | 23.53ab | 20.34dc | 0.66b | 0.37b |
| 66.66 | 4.54 | 14.77ab | 10.43f | 3.21c | 23.81a | 20.63d | 0.57c | 0.28d |
| 0 | 6.81 | 13.54cd | 13.34d | 3.25b | 19.33de | 21.33cd | 0.68ab | 0.16g |
| 22.22 | 6.81 | 14.35b | 14.52b | 3.53b | 20.42cd | 22.05bcd | 0.69ab | 0.21f |
| 44.44 | 6.81 | 14.27bc | 14.32bc | 3.55a | 21.38c | 21.63c | 0.57c | 0.32c |
| 66.66 | 6.81 | 15.66a | 7.61gh | 3.11cd | 22.43b | 24.44a | 0.72a | 0.35bc |
| Mean | 13.42 | 12.79 | 3.3 | 21.63 | 20.9 | 0.62 | 0.3 | |
| CV | 10.45 | 9.31 | 6.88 | 5.72 | 4.96 | 6.33 | 6.77 | |
Table 2: The combined effect of different concentration of BAP and TDZ on morphogenetic response of shoot growth parameters of Bako local pepper variety cultured on full strength MS medium using shoot tip as explant.
The combinations effect of different concentration of BAP and TDZ with IBA on shoot induction and multiplication of Marko fana and Bako local pepper varieties
The result data presented in Table 3 showed significant differences on mean shoot number at the combination of BAP and TDZ with IBA. Chandra, et al. repot indicated shoot proliferation directly from shoot tip explants on MS media supplemented with 22.2 μM of BAP combined with 2.5 μM-4.9 μM of IBA and obtained significantly maximum mean number of shoots (15.23 per explant) in Capsicum annum L.cv. Mathania. In the present study, for the variety Marko fana significantly maximum mean number of shoots (18.36 per explant) were proliferated on MS medium supplemented with 66.66 μM of BAP combined with 4.9 μM of IBA. When the explants cultured on MS media fortified with the combination of 6.81 μM of TDZ with 4.9 μM of IBA, highly significant mean number of shoots (21.25 per explant) were recorded. On the Bako local pepper variety, significantly maximum mean number of shoots per explant (19.62) were obtained in explants cultured on MS media fortified with the combination of 6.81 μM of TDZ with 4.9 μM of IBA followed by 4.54 μM TDZ combined with 7.35 μM of IBA (17.84). This result also revealed that better shoot proliferations were obtained at the combination of TDZ with IBA compared to BAP with IBA combinations. Increasing the concentrations of IBA to MS medium significantly decreased the proliferation of shoots. However, addition of optimal concentration of BAP and TDZ to the MS medium, the proliferation of shoots per explant was increased in both pepper varieties.
Peddaboina, et al. reported that multiple shoot elongation occurred upon transfer to 9.77 μM BA combined with 4.8 μM of IAA. However, in the present study as results indicated in Table 3, revealed that 66.66 μM of BAP combined with 4.9 μM of IBA gave highly significant maximum mean shoots length (4.27) which is the best and optimum for the variety Marko fana compared with other hormonal combinations. When the explants were cultured on MS media treated with 6.81 μM of TDZ combined with 4.9 μM of IBA, significantly maximum mean length of shoots (3.78) were induced. On the other hand, the combinations having 66.66 μM of BAP and 6.81 μM of TDZ with 4.9 μM of IBA produced the best height (3.83) and (3.35) for the Bako local pepper variety, respectively. This result also revealed the combination of BAP and IBA gave best shoot length compared to TDZ with IBA combinations.
Sharma, has been reported the effectiveness of TDZ alone or in combination with IBA in pepper tissue cultures. The report of Kumar, et al. revealed that 26.63 μM BAP combined with 2.28 μM IAA were found to be optimum for shoot bud induction by producing 19 shoot buds and 24 shoot buds in Arka Abhir and Arka Lohit pepper varieties, respectively. Similarly, in the present study for the variety Marko fana significantly maximum mean number of shoot buds per explant (25.45) were differentiated on MS medium supplemented with 6.81 μM of TDZ combined with 4.9 μM of IBA. According to the report of Sanatombi and Sharma, indicated that MS medium containing 8.8 μM BAP combined with 11.4 μM IAA was found to be the best medium for shoot bud induction and produced maximum mean number of shoot buds (14.4) per explant for Capsicum annuum cv. Meiteimorok. However, in the present experiment, for the variety Bako local significantly maximum mean numbers of buds (25.23) per explant were obtained at the combination of 4.54 μM of TDZ with 7.3 μM of IBA followed by 23.82 mean shoot buds at the combination of 6.81μmol/l of TDZ with 4.9 μM of IBA. Shoot bud differentiation increased with increasing concentration of TDZ up to 6.81 μM of TDZ and decreasing the concentration of IBA up to 2.45 μM. Some earlier reports of in vitro studies in pepper also reported the effectiveness of BAP in combination with IBA in inducing multiple shoot buds in pepper tissue cultures and this result also suggest the same and explants cultured on MS media treated with 66.66 μM of BAP combined with 4.9 μM of IBA, significant mean number of shoot buds (22.67) and (21.42) were differentiated for Marko fana and Bako local pepper varieties respectively.
In case of mean number of leaves per explant, the result presented in Table 3 revealed that there was slight significant difference between varieties and among treatments. In the variety Marko fana significantly maximum mean number of leaves (28.63) were found in explants cultured on full strength MS media supplemented with 6.81 μM of TDZ combined with 4.9 μM of IBA followed by 24.43 leaves per explant at the combination of 66.66 μM of BAP with 4.9 μM of IBA compared with other hormonal combinations.
In contrast, significantly minimum mean numbers of leaves (18.33) were obtained at the combination of 9.8 μM of IBA with 22.22 μM of BAP. The number of leaves proliferated per explant was increased at higher concentrations of BAP and TDZ with low concentration of IBA but not vice versa. For the variety Bako local, significantly maximum mean number of leaves per explant (26.34) were found in explants cultured on MS media supplemented with 4.54μM of TDZ combined with 7.35 μM of IBA followed by 6.81 μM of TDZ combined with 4.9 μM of IBA (24.33 leaves). When the explants cultured on MS media fortified with 66.66 μM of BAP combined with 4.9 μM of IBA, (23.64 leaves per explant) were obtained. However, minimum mean numbers of leaves (16.66) per explant were obtained at the combination of 9.8 μM of IBA with 22.22 μM of BAP. This result also showed the combination of TDZ with IBA was best and optimal for leave proliferation compared to BAP with IBA combinations. Generally, as the concentration of IBA increased, the proliferation of leaves per explant decreased but when the concentration of BAP and TDZ were increased up to their optimum levels than the concentration of IBA, well proliferation of leaves were obtained in both tested pepper varieties.
The present study showed significant biomass differences among treatments and between varieties in the combination of BAP and TDZ with IBA. In the variety Marko fana highly significant mean shoot fresh weight (0.72 g) and shoot dry weight (0.56 g) per explant was recorded in explants cultured on full strength MS medium treated with 6.81 μM of TDZ combined with 4.9 μM of IBA. When the explants cultured on MS media fortified with 66.66 μM of BAP combined with 4.9 μM of IBA, significant mean shoot fresh weight (0.65g) and shoot dry weight (0.45g) were recorded. In contrast, significantly minimum mean numbers of shoot fresh weight (0.36 g) and shoot dry weight (0.31 g) per explant were obtained at the combination of 9.8 μM of IBA with 2.27 μM of TDZ. On the Bako local pepper variety, significantly maximum mean shoot fresh weight (0.70 g) and shoot dry weight (0.52 g) per explant were obtained at the combination of 4.54 μM of TDZ with 7.35 μM of IBA followed by (0.66 g) and (0.47 g) at the combination of 6.81 μM of TDZ with 4.9 μM of IBA respectively.
| Pepper varieties | Plant growth regulators In (µM) | Shoot growth parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BAP | TDZ | IBA | Mean no. of shoots/exp (n) | Mean shoot length/exp (cm) | Mean no. of buds/exp (n) | Mean no. of leaves/exp (n) | Mean shoot Fw/exp(g) | Mean shoot Dw/exp (g) | |
| Marko Fana | 22.22 | 0 | 9.8 | 13.22d | 2.22ab | 14.02e | 18.33ef | 0.38e | 0.32e |
| 44.44 | 0 | 7.35 | 16.51c | 3.51cd | 16.51d | 22.75c | 0.51d | 0.36cd | |
| 66.66 | 0 | 4.9 | 18.36b | 4.27a | 22.67b | 24.43b | 0.65b | 0.45b | |
| 88.88 | 0 | 2.45 | 17.53bc | 3.11b | 19.81c | 20.62d | 0.47bc | 0.31ef | |
| 0 | 2.27 | 9.8 | 9.41f | 2.43cd | 13.75ef | 19.32e | 0.36f | 0.31e | |
| 0 | 4.54 | 7.35 | 11.33e | 2.75b | 20.51c | 21.75d | 0.53cd | 0.34d | |
| 0 | 6.81 | 4.9 | 21.25a | 3.78b | 25.45a | 28.63a | 0.72a | 0.56a | |
| 0 | 9.08 | 2.45 | 15.30c | 1.42c | 17.51d | 21.41d | 0.55c | 0.39c | |
| Mean | 15.36 | 2.94 | 18.78 | 22.16 | 0.52 | 0.38 | |||
| CV (%) | 8.55 | 4.78 | 12.32 | 9.88 | 7.91 | 6.25 | |||
| Bako Local | 22.22 | 0 | 9.8 | 10.47e | 1.55f | 12.64g | 16.66f | 0.35g | 0.23g |
| 44.44 | 0 | 7.35 | 13.34d | 2.97d | 18.33e | 18.51e | 0.38fg | 0.25f | |
| 66.66 | 0 | 4.9 | 15.23c | 3.83a | 21.42c | 23.63b | 0.61c | 0.43b | |
| 88.88 | 0 | 2.45 | 16.64bc | 2.44e | 20.54d | 20.44d | 0.54d | 0.38c | |
| 0 | 2.27 | 9.8 | 7.75f | 2.12ef | 14.75f | 15.23fg | 0.48e | 0.33d | |
| 0 | 4.54 | 7.35 | 17.84b | 3.21c | 25.23a | 26.34a | 0.70a | 0.52a | |
| 0 | 6.81 | 4.9 | 19.62a | 3.35b | 23.82b | 24.33b | 0.66b | 0.47b | |
| 0 | 9.08 | 2.45 | 13.32d | 1.43fg | 18.38e | 19.55de | 0.42f | 0.31e | |
| Mean | 14.28 | 2.61 | 19.43 | 20.59 | 0.54 | 0.36 | |||
| CV (%) | 9.24 | 5.84 | 11.52 | 10.15 | 6.72 | 7.65 | |||
Table 3: The combinations effect of BAP and TDZ with IBA on shoot growth parameters of Marko fana and Bako local varieties.
The combined effect of auxins on in vitro root growth parameters of Marko fana and Bako local pepper varieties
The combined effect of IBA and NAA on root induction of Marko fana and Bako local pepper varieties cultured on full MS media: The result data presented in Table 4 indicated that the different combination of IBA and NAA exhibited statistically significant variations on root growth parameters (number of roots, root length and root fresh and dry weight) among treatments and between varieties due to hormonal as well as PGRs with varietal interactions. The report of Ashrafuzzaman, et al. revealed the effectiveness of root initiating hormonal combination on root regeneration of pepper and in his study the mean number of roots per shoot varies from 8.8-12.0 in shoot tip derived shoot and he obtained maximum mean number of roots (12.0) per shoot on MS media supplemented with 5.3 μM NAA plus 4.9 μM IBA. However, in the present study 4.9 μM of IBA combined with 2.65 μM of NAA gave highly significant.
Maximum mean number of roots (38.21) per explant followed by 2.45 μM of IBA combined with 2.65 μM of NAA (35.27) for the variety Bako local. Whereas 7.35 μM of IBA combined with 2.65 μM/l of NAA gave maximum mean number of roots per explant (35.52) including the combination of 4.9 μM of IBA with 5.3 μM of NAA (33.25 roots) for the variety Marko fana. Among the two hormone combinations, the combinations having 4.9 μM and 7.35 μM of IBA with 2.65 μM of NAA produced the best number of roots for the variety Bako local and Marko fana respectively. Concerning the length of roots, the different IBA and NAA combinations results revealed significant variation in mean root length among treatments and between varieties. The variety Marko fana induced significantly maximum mean length of root (3.47 cm) at the combination of 7.35 μM of IBA with 2.65 μM of NAA followed by 3.42 cm at the combination of 2.45 μM of IBA with 5.3 μM of NAA. On the other hand, the variety Bako local induced highly significant maximum mean length of root (3.62 cm) at the combination of 2.45 μM of IBA with 2.65 μM of NAA compared with individual treatments of 2.65 μM of NAA (3.56 cm) and 4.9 μM of IBA (3.22 cm). The analysis of variance presented in Table 4, revealed that the root fresh and dry weight were statistically significant between varieties as well as among the combined auxin treatments. Significant maximum mean root fresh weight (0.76 g) and root dry weight (0.42 g) in the combination of 4.9 μM of IBA with 2.65 μM of NAA were recorded for the variety Bako local. On the variety Marko fana, the highest root fresh weight and root dry weight value per explant were obtained for explants cultured on full strength MS medium supplemented with the combination of 7.35 μM of IBA with 2.65 μM of NAA (0.39 g) followed by (0.65 g) and (0.37 g) at the combination of 4.9 μM IBA with 5.3 μM NAA respectively.
| Pepper varieties | Auxins in (µM) | Mean days for root emergence | Mean no. of roots/exp (n) | Mean root length/exp (cm) | Mean root Fw/exp (g) | Mean root Dw/exp (g) | |
|---|---|---|---|---|---|---|---|
| IBA | NAA | ||||||
| Marko Fana | 7.35 | 0 | 12.35cd | 26.33c | 3.24cd | 0.53c | 0.26e |
| 7.35 | 2.65 | 3.56bc | 35.52a | 3.47a | 0.72a | 0.39a | |
| 7.35 | 5.3 | 12.33d | 27.42c | 2.84d | 0.45d | 0.31d | |
| 7.35 | 7.95 | 14.57b | 20.55d | 2.75e | 0.32e | 0.22f | |
| 0 | 5.3 | 12.67c | 31.33bc | 3.31c | 0.51cd | 0.32d | |
| 2.45 | 5.3 | 11.96e | 32.51b | 3.42b | 0.54c | 0.35c | |
| 4.9 | 5.3 | 3.39bc | 33.25b | 3.11cd | 0.65b | 0.37b | |
| 7.35 | 5.3 | 15.34a | 27.42c | 2.84d | 0.51cd | 0.31d | |
| Mean | 13.27 | 29.29 | 3.12 | 0.53 | 0.32 | ||
| CV (%) | 8.65 | 7.34 | 8.25 | 15.37 | 16.12 | ||
| Bako Local | 4.9 | 0 | 12.66d | 25.33d | 3.22d | 0.47c | 0.28c |
| 4.9 | 2.65 | 13.42c | 38.21a | 3.45c | 0.76a | 0.42a | |
| 4.9 | 5.3 | 12.23e | 33.84bc | 2.95e | 0.54bc | 0.29c | |
| 4.9 | 7.95 | 14.49b | 28.45c | 2.74ef | 0.44c | 0.21d | |
| 0 | 2.65 | 14.35b | 34.33bc | 3.56b | 0.36d | 0.15e | |
| 2.45 | 2.65 | 13.22bc | 35.27b | 3.62a | 0.57b | 0.38b | |
| 4.9 | 2.65 | 13.42c | 38.21a | 3.45c | 0.76a | 0.42a | |
| 7.35 | 2.65 | 15.27a | 29.71c | 2.67f | 0.49c | 0.23d | |
| Mean | 13.63 | 32.92 | 3.21 | 0.55 | 0.29 | ||
| CV (%) | 9.31 | 5.95 | 6.87 | 13.56 | 18.45 |
Table 4: The combinations effects of different IBA and NAA on root growth parameters of Marko fana and Bako local pepper varieties cultured on full MS media.
Virus indexing
The DAS-ELISA result showed that among the tested 60 samples of Marko fana pepper variety 86% (52 individuals) of the samples were positive for three pepper infecting viruses i.e. 36% (22 individuals) for EPMV, 32% (19 individuals) for PVY and 18% (11 individuals) for PVMV and the remaining 14% (8 individuals) of the samples were negative. However when the apical shoot tips of the 86% of virus positive samples were surface sterilized and propagated in vitro and tested for the presence of these viruses via DAS-ELISA test, all of the in vitro propagated plantlets became totally free (negative). On the other hand, the Bako local pepper variety was less susceptible for the tested three pepper infecting viruses compared to Marko fana pepper variety and among the tested 60 samples of Bako local pepper variety, 68% (41 individuals) of the samples were positive for the three pepper infecting viruses i.e. 28% (17 individuals) for EPMV, 23% (14 individuals) for PVY and 17% (10 individuals) for PVMV and the remaining 32% (19 individuals) of the samples became free (negative). When the apical shoot tip of the 68% of virus positive samples were surface sterilized and propagated in vitro and tested the in vitro regenerated plantlets via DAS-ELISA, all of these viruses were eliminated. This result confirmed that shoot tip tissue culture is effective in virus clearance from virus infected pepper crops. The anti-sera reaction for PVY and EPMV was strong (deep yellow color developed as substrate added quickly (Figure 1C and 1E, respectively). The anti-sera reaction for PVMV was weak (not deep yellow) but yellower than that of negative control (Figure 1). Generally, this experiment showed not only the eradication of pepper viruses via shoot tip tissue cultures from virus infected pepper varieties but also ensure the occurrence of three kinds of viruses were tested in Marko fana and Bako local pepper varieties.
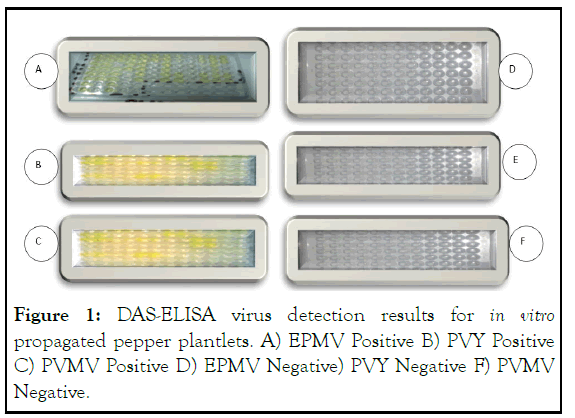
Figure 1: DAS-ELISA virus detection results for in vitro propagated pepper plantlets. A) EPMV Positive B) PVY Positive C) PVMV Positive D) EPMV Negative) PVY Negative F) PVMV Negative.
Acclimatization
In vitro induced shoots are very delicate and prone to sudden environmental changes that may damage the plantlets unless it is gradually adapted to the new environment. Thus, acclimatization is essential to enable the rooted plantlets to adapt the natural environment in ex-vitro conditions. In the acclimatization stage of this experiment, a total of 112 well rooted shoot lets (56 shootlets from each pepper varieties) from each treatments of plant growth regulators were transferred to pots containing either sand alone, moist red soil alone, compost alone or a mixture of the three culture substrates at the ratio of 2:1:1 and acclimatized for one week by irrigating water and aeration daily to maintain the humidity of the plantlets.
The acclimatization phase of this experiment revealed that the effect of PGR and culture substrates showed significant differences on the survival of the in vitro regenerated plantlets between varieties as well as among treatments. Among the tested four acclimatization culture substrates, the mixture of sand, moist red soil and compost in the ratio of 2:1:1 was showed highly significant differences on the survival of in vitro regenerated pepper plantlets. At this mixture of culture substrates, it showed significantly maximum (81.25%) and (70.59%) plantlet survival for Marko fana and Bako local pepper varieties, respectively. This result also revealed the highest survival rate on the variety Marko fana plantlets as compared to Bako local pepper variety (varietal difference).
Among the tested cytokinins the highest percentage of plantlet survival was observed on Marko fana that was transferred from full strength MS media supplemented with 44.44 μM/l of BAP and 6.81 μM/l of TDZ. Whereas the highest survival for Bako local pepper variety was shown on the MS media treated with 66.66 μM/l of BAP and 4.54 μM/l of TDZ (treatment difference) (data not shown). Better survival and acclimatization for longer shoots is in accordance with the recommendations of Peddaboina, et al. Moreover, the essentiality of appropriate root development in vitro for successful establishment of pepper shootlets during acclimatization was agrees with Sanatombi and Sharma. The report of Venkataiah showed that Plantlets obtained from TDZ-containing media were normal diploid (2n=24) and could readily be established in the soil under greenhouse conditions with a survival frequency of 68%-84% (Table 5).
| Types of peppervarieties | Types of culture substrates | Total No. of explants transferred | No. of explants survived | No. of explants died | % of explants survived | % of explants died |
|---|---|---|---|---|---|---|
| Marko Fana | Sand alone | 12 | 6d | 6a | 50.00d | 50.00a |
| Moist red soil alone | 13 | 7c | 6a | 53.85c | 46.15b | |
| Compost alone | 15 | 11b | 4b | 73.33b | 26.67c | |
| Mixture in 2:1:1 ratio | 16 | 13a | 3c | 81.25a | 18.75d | |
| Mean | 14 | 9.25 | 4.75 | 60.44 | 39.56 | |
| CV | 8.45 | 7.55 | 6.83 | 5.22 | 6.52 | |
| Bako local | Sand alone | 11 | 4d | 7b | 36.36d | 63.64a |
| Moist red soil alone | 13 | 5c | 8a | 38.46c | 41.54b | |
| Compost alone | 15 | 9b | 6c | 60.00b | 40.00c | |
| Mixture in 2:1:1 ratio | 17 | 12a | 5d | 70.59a | 29.41d | |
| Mean | 14 | 7.5 | 6.5 | 51.35 | 43.65 | |
| CV | 8.45 | 6.92 | 7.03 | 5.71 | 5.92 |
*Means with same letter (s) in the same column are not significantly different at p<5% significance level
Table 5: The effect of different culture substrates on the survival of in vitro regenerated plantlets of Marko fana and Bako local pepper varieties during acclimatization.
Pepper (Capsicum annum L.) is an economically important vegetable and spice crops that belonging to the genus Capsicum and the family Solanaceae. The world pepper demand for the use as vegetable and spice has consistently increased. However, the production of pepper has been generally low over the last several years mainly due to the vulnerability of the pepper genotypes to a multitude of abiotic (extreme temperature, light, moisture, nutrients and pH) and biotic stresses (various fungi, bacteria and viruses diseases).
Conventional propagation methods of pepper through seeds require large amount of materials and space for propagation and an extended period to produce plants. It also further restricted by short life span of seed viability and low germination rate of the seeds. These limitations prevent an efficient and rapid production of pepper to meet the current market demand of pepper. Thus in vitro propagation is recommended to assist the conventional method. Since the plants also lack natural vegetative propagation, tissue culture methods provide a novel way for the asexual multiplication of pepper plants. In vitro plant regeneration from cells, tissues and organ cultures is a fundamental process for the application of biotechnology to propagation, breeding and genetic improvement of pepper. However, application of cell and molecular biology techniques to pepper genetic improvement is limited because of the difficulties in efficient pepper plant regeneration protocols. For pepper plant breeding using somaclonal variation and gene transformation of useful traits, such as virus-resistance, an efficient pepper in vitro regeneration protocols are needed.
Therefore, to overcome these problems and to fulfill the demand for large-scale cultivation in a short period by rapid mass multiplication, an experiment was conducted with the aim of developing a protocol for the in vitro propagation of pepper from in vitro seedling and green house grown pepper seedling explants. The experiment was carried out at the plant tissue culture laboratory of Melkassa agricultural research center. The protocol involves four subsequent experiments: in vitro shoot induction, rooting of the in vitro proliferated shoots, virus indexing and acclimatization of the regenerated plantlets under ex vitro condition. For the shoot induction experiment, juvenile shoot tip (0.5 cm) explants were cultured on MS medium contained 30 gm/l sucrose, 7 gm/l agar, vitamins and supplemented with cytokinins with the combinations (44.44 μM and 66.66 μM of BAP with 0, 2.27 μM, 4.54 μM and 6.81 μM of TDZ) and (4.54 μM and 6.81μM of TDZ with 0 μM, 22.22 μM, 44.44 μM and 66.66 μM of BAP). The analysis of the results indicated that there was a significant difference among the treatments as well as between varieties interims of shoot growth parameters. The two cytokinins combinations, 4.54 μM of TDZ and 6.81 μM of TDZ with 66.66 μM of BAP for the variety Bako local and 44.44 μM of BAP with 4.54 μM of TDZ and 6.81 μM of TDZ for the variety Marko fana were resulted the best performance in shoot growth parameters during shoot induction experiment. For the root induction experiment, full strength MS media fortified with auxins the two combinations for each pepper varieties (7.35 μMof IBA with 0 μM, 2.65 μM, 5.3μM and7.95 μMofNAA and 5.3 μM of NAA with 0 μM, 2.45 μM, 4.9 μM and 7.35μM of IBA for the variety Marko fanaand4.9 μM of IBA with 0, 2.65 μM, 5.3 μM and 7.95 μM ofNAA and 2.65 μM/l of NAA with 0 μM, 2.45 μM, 4.9 μM and 7.35 μM of IBA for the variety Bako local) were thoroughly examined. This result revealed that among the tested auxin combinations, 2.45 μM and 4.9 μM of IBA with 2.65 μM of NAA for the variety Bako local and 4.9 μM of IBA with 5.3 μM of NAA as well as 7.35 μM of IBA with 2.65 μM of NAA for the variety Marko fana were resulted significantly best performance in root growth parameters.
In the virus indexing experiment, in the two pepper varieties, both the mother plant before the experiment and the in vitro regenerated plantlets after the experiment were tested for three types of pepper viruses (PVY, EPMV and PVMV) by DAS-ELISA test. A total of 120 samples for the two pepper varieties (60 sample for Marko fana and 60 samples for Bako local variety) were tested and used the positive results as a mother plant. The DASELISA result showed that among the tested 60 samples of Marko fana pepper variety 86% of the samples were positive for three viruses (36% of EPMV, 32% of PVY and 18% of PVMV) and only 14% of the samples were negative. However when the 86% of virus positive samples apical shoot tips were surface sterilized and cultured in vitro and tested for the presence of these viruses all in vitro propagated plantlets became totally free (negative). On the other hand, among the tested 60 samples of Bako local pepper variety 68% of the samples were positive for three pepper viruses (28% of, 23% of PVY and 17% of PVMV) and the remaining 32% of the samples were free (negative). When the apical shoot tip of the 68% of virus positive samples were surface sterilized and propagated in vitro and tested the in vitro regenerated plantlets via DAS-ELISA, all of these viruses were eliminated. This result also showed that Marko fana pepper variety was more susceptible for the tested three pepper viruses compared to Bako local pepper variety.
In the acclimatization experiment, the effect of PGRs and different culture substrates on the survival of in vitro propagated pepper plantlets were examined by transferring well rooted in vitro propagated pepper shootlets from each treatments of plant growth regulators to pots containing either sand alone, moist soil alone, compost alone or a mixture of the three culture substrates at the ratio of 2:1:1 and acclimatized for one week by irrigating water and aeration daily. Among the tested four acclimatization culture substrates, the mixture of sand, moist red soil and compost in the ratio of 2:1:1 showed significantly maximum survival (81.25%) and (70.59%) of in vitro regenerated pepper plantlets for Marko fana and Bako local pepper varieties respectively. Among the tested cytokinins significant plantlet survival was observed on Marko fana that was transferred from MS media supplemented with 44.44 μM of BAP and 6.81 μM of TDZ. Whereas the highest survival for Bako local pepper variety was shown on the MS media treated with 66.66 μM of BAP and 4.54 μM of TDZ.
Thus the present study in vitro propagation and virus elimination by shoot tip culture of pepper found in Ethiopia is the first of its kind. This preliminary study was under taken to optimize the duration of the sterilization time and plant growth regulators for shoot and root induction as well as virus elimination by apical shoot tip tissue culture from virus infected two commercially released pepper varieties (Marko fana and Bako local). Based on these studies:
• In the present study an efficient and reliable in vitro propagation protocols were developed for Marko fana and Bako local pepper varieties.
• In the surface sterilization experiment, immersing the explants by 5% chlorine containing berekina for 20 minutes and 3%consider with 2 drops-3 drops of Tween-20 for 15 minutes was found to be effective for surface sterilization of the pepper plant.
• The cytokinins combinations, 4.54 μM of TDZ and 6.81 μM of TDZ with 66.66 μM of BAP for the variety Bako local and 44.44 μM of BAP with 4.54 μM of TDZ and 6.81 μM of TDZ for the variety Marko fana were resulted the best performance in shoot growth parameters during shoot J Agri Sci Food Res, Vol 14. Iss.2 No:1000143 (MRPFT) 14 induction experiment.
• For this study the result revealed that among the tested auxin combinations, 2.45 μM and 4.9 μM of IBA with 2.65 μM of NAA for the variety Bako local and 4.9 μM of IBA with 5.3 μM of NAA as well as 7.35 μM of IBA with 2.65 μM of NAA for the variety Marko fana were resulted significantly best performance in root growth parameters.
• In vitro propagation techniques such as shown in the present study was economically effective to solve the problem associated with the conventional propagation of pepper.
• Since various diseases, especially viral diseases, affect pepper, virus indexing techniques (DAS-ELISA) were conducted in this study to confirm the efficiency of shoot tip tissue culture in elimination of pepper infecting viruses.
• In the acclimatization process both varieties have good survival rate. However, Marko fana that was cultured on 66.66 μM of BAP and 4.54 μM of TDZ showed highest survival rate than Bako local variety cultured on the same plant regulators.
In conclusion, it is beneficial to use the in vitro propagation protocols developed in this study and it presents an efficient system of in vitro clonal propagation compared to seed propagation for rapid multiplication, production of disease-free plants, non-seasonal production, germplasm conservation and facilitating their easy exchange to overcome the challenges of conventional cultivation of pepper. Based on this study it is recommended that since, the present study was done for only two pepper varieties; it would be advisable to repeat the experiment for other pepper varieties and further studies must focus on the performance of the in vitro propagated pepper plantlets for growth characters, yield and yield related parameters as well as yield quality parameters in field establishment conditions in order to arrive at a sound conclusion.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Belete DA (2023) Determination of the Combined Effect of Different Concentration of Cytokines and Auxins on in-vitro Shoots and Root Growth Parameters of Pepper (Capsicum annum L.) Varieties through Shoot Tip Culture. J Agri Sci Food Res. 14:143.
Received: 02-Nov-2022, Manuscript No. JBFBP-22-19924; Editor assigned: 04-Nov-2022, Pre QC No. JBFBP-22-19924 (PQ); Reviewed: 18-Nov-2022, QC No. JBFBP-22-19924; Revised: 10-Jan-2023, Manuscript No. JBFBP-22-19924 (R); Published: 17-Jan-2023 , DOI: 10.35248/2593-9173.23.14.147
Copyright: © 2023 Belete DA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.