Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2013) Volume 3, Issue 2
In the present study the dissolution of calcium hydroxide (Ca(OH)2 in water as well as in mixed solvents systems such as (methanol + water), (ethanol + water), (1-propanol + water) and (2-propanol + water) in 0-25 percentage compositions range at 293 ± 1K and 343 ± 1K or (328 ± 1K in case of methanol) was undertaken for the determination of solubility, solubility product constant (Ksp),Gibbs free energy change (ΔGo), entropy change (ΔS°) and enthalpy change (H°) by pH-metric method. It was found that the equivalence point was decreased linearly by the increasing of the percentage composition of the mixed solvents systems. This represents that solubility and KSP values are linearly decreased. The solubility of Ca(OH)2 was decreased at higher temperature compared to room temperature. Furthermore, methanol produced minimum decreased due to its high value of dielectric constant while in 2-propanol showed maximum decreased in solubility and KSP values. On the other hand Go values were linearly increased against percentage composition of mixed solvents systems. Entropy and Enthalpy values were also changed in mixed solvent systems.
<Keywords: Thermodynamic parameters; Calcium hydroxide; Mixed solvents; Dielectric constant
Calcium hydroxide is used in a broad range of industrial, professional and consumer applications. Calcium hydroxide is used as such or in a mixture for the production of articles to be used in or for vehicles, construction, electronic apparatus, laboratories, fabrics, wood, rubber, plastics, metal, leather, chemicals, pharmaceuticals, pesticides , the treatment of potable water, waste water, municipal sludge cosmetics, personal care products [1-5].
The solubility of solutes in mixed solvents is of great practical importance since many industrial process as well as laboratory procedures call for the use of solvent mixtures. The solubility of solutes in mixed solvents depends primarily on the solvation of solutes or their constituent ions by the components of solvent mixtures [6]. Studying the thermodynamics of different salts, is important for evaluating the single ion thermodynamic parameters which help in explain the preferential solvation of the ions [7].
There are various ways for determination of thermodynamic parameters suchGibbs free energy change (ΔG°), entropy change (ΔS°) and enthalpy change (ΔH°). These parameters state the physiochemical properties of a chemical system. Previously, many techniques have been applied to find the solubility products of various systems. These techniques include spectral studies, viscosity, refractive index and some electro-analytical method like conductometry, potentiometry, polarography and voltametry etc. Most of the researchers aimed to infer data of very precise solubility product of these systems that are extremely less soluble or nearly insoluble in aqueous systems [8-10]. Method of very precise evaluation of Ksp values of various types of reagents having structural dissimilarities are reported [11]. Clark also suggested a method to evaluate solubility product constant for various compounds along with a practical aspect pertaining to the problems associated with the determination of such compounds [12]. A straight forward method was published showing determination of various thermodynamic parameters such as ΔG°, ΔS° and ΔH° for the dissolution of calcium hydroxide in aqueous by simple titration method [13].
Different properties such as solubility, Ksp, Gibbs free energy, entropy and enthalpy associated with sparingly soluble salts are influenced with different parameters, such as change in percentage of solvent, amount of salts and temperature etc. [14-16].
In the present research work, pH metric method was used for the study of the influence of mixed solvent system on the solubility Ksp, Gibbs free energy, entropy and enthalpy of Ca(OH)2.
When a sparingly soluble compound like Ca(OH)2 is added into water, equilibrium is established between the solid and aqueous material that can be approximately represented by the equation [13].
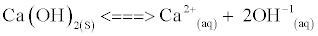 (1)
(1)
The molar solubility “S” solubility product and Gibbs free energy is determined from following equations [13].
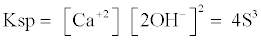 (2)
(2)
ΔG = -R T l n (Ksp) (3)
Where “R” is a universal gas constant, T is the absolute temperature and ΔG° is the Gibbs free energy ΔG° is related to ΔH° and ΔS° by the following equation [13].
ΔG°= H°- TΔS° (4)
The value of ΔG° calculated at two different temperatures T1 and T2 provide the values of ΔH° and S° over a small temperature range.
In previous work conductometric method was used for determination of thermodynamic parameters in mixed solvent systems [17]. But in present work pH metric method has been used.
In the present research work, pH –metric method was used for the study of the influence of mixed solvent system on the solubility of Ca(OH)2. All reagents were used such as Ca(OH)2 (E. Merck), 1-Propanol (E. Merck), 2-propanol (E. Merck), methanol (E. Merck), ethanol (E. Merck), Hydrochloric acid (E. Merck), Oxalic acid (E. Merck), Sodium hydroxide (E. Merck), Phenolphalein (E. Merck) and Buffer solutions (E. Merck) each of these analytical grade reagents. These stock solutions were prepared in double distilled water. The equipment used were pH meter (Mettler Toledo MP 220) Japan, IKA Combimag RCH magnetic stirrer (Germany) Thermostat (Chiller) Haake, (Type T 52, V 220, No 76400, Germany). The thermostate environment (0 -110 ± 0.5°C) and Electrical Balance (Mettler college 150, Germany).
Preparation of stock solution
The pH metric titration was performed in order to determine the solubility of Ca(OH)2, at 293, and 343 ± 1K. The following procedure of was followed. Weighed 0.1 g of calcium hydroxide was transferred to a 100 ml volumetric flask andadded 0, 1, 2, 3, 4, 5, 6, 10, 15, 20 and 25 percentages of mixed solvents (1-propanol, 2-propanol, methanol and ethanol and then made up the mark with water. Stirred for 30 minutes and then this solution were kept for 24 hours in thermostat at fixed temperatures to get maximum saturation. This sample is filtered using Whatman filter paper 60°A. A 25.00 mL aliquot is transferred to a 100- mL beaker. The sample is titrated with standardized hydrochloric acid 0.03 ± 0.001 M) and pH was recorded during the titration. The reacting mixture flask was placed in water bath during the whole titration and temperature was controlled by thermostat at a fixed temperature. The titration is repeated three times. The neutralized solution was washed down to drain [18]. The equivalence point was obtained by 1st derivative curve. The pH meter was calibrated with buffer solution of pH 4, 7 and 10. The pH meter showed zero error against the buffers.
In mixed solvent systems the pH of Ca(OH)2 was gradually decreased by the addition of each volume (ml) of Standard HCl (0.03 ± 0.001 M) by the mutual exchange of H+ and OH ions from acid with analyte after the equivalence point, pH of mixture is decreased sharply in increasing the H+ ion in solution. In all these cases the pH variation started from 12.99 ± 0.01 to 2.62 ± 0.02. The titration curves were shown in Figure 1.
The decrease in equivalence point while plotting against the varied composition of these mixed solvent systems (0-25 percentages) is represented in Figure 2. The various mixed solvents influenced in equivalent point into different extents depending upon their dielectric constant, solvation behaviors and interaction with water and solute. The methanol+water system showed least variation tendency in equivalence point while 2-propanol+ water showed maximum effect as compared to the equivalence point in pure water at 293 ± 1K and 343 ± 1K. However the dissolution of Ca(OH)2 was decreased slightly by increasing temperatures. The reversed trend in dissolution against temperature, in Ca(OH)2 was found due to its lattice energy. The solvation of ion was possibly influenced by temperature in two ways. For instance it has been reported that ions- ions interaction is usually decreased by increasing temperature that results in the solvation of ions which enhances by rise in temperature [11,17,19-21]. However, the solvation of ions may inversely be influenced by the rise in temperature, were a possible decrease in solvation occurs with the rise in temperature.
The solubility of calcium hydroxide was slightly decreased by increasing the % composition of mixed solvent systems as compare to pure water shown in the Figure 3 and 4. The decreasing trend in the solubility was minimum up to 5 percentage composition in all the mixed solvent systems whereas, a significant decrease occurred from 6-25 percentage compositions of mixture solvents. The methanol + water system showed minimum decreasing trend while 2–propanol + water system showed maximum at both temperatures. The reason of such behavior is the nature of dielectric constant, extent of solvent– solvent interaction and solvent–solute interaction, which is reported in literature [22-24]. The dielectric constant is the ability of any solvent, which reduces the forces between solute ions. Since the dielectric constant of water is 80, which is greater than that of any solvent so it reduces the force of attraction between solute ions, as a result ions are separated and solute is dissolved. Therefore, solubility of any salt is greater in water than in the other solvents. The solvents selected in the present research work have the following order of dielectric constant values water>methanol >ethanol >1-propanol >2-propanol [14,25]. As the percentage compositions of these solvents increased in water the values of dielectric constant decreases as a result solubility also decreases.
Solubility product values of Calcium hydroxide, in pure aqueous system as well as in mixed solvent systems like (methanol-water, ethanol –water, 1-propanol-water and 2-propanol water) were calculated from their solubility values from the equation 2 and represented in Table 1 and 2. The Figure 5 showed that a slightly non-linear decreasing trend with least decrease in Ksp values was observed in all mixed solvent systems up to their 5% composition against pure water, however from 6%-25% compositions in the presence of mixed solvent systems, a decrease in their Ksp values occurred. The reason of non-linearity in the Ksp values is due to the term of product of solubility, which altered the behavior in a non-linear fashion (having based Ksp =4s3).
The ΔG° values for the dissolution of Calcium hydroxide in water and mixed solvent system at two different temperatures were evaluated from the Ksp values calculated by equation 3 and presented in Table 3 and 4. The Figure 6 showed that the ΔG° values were increased linearly with increase in percentage compositions of mixed solvent systems. This trend was found similar in both temperatures. It was found that the methanol system showed minimum increase in ΔG° values while 2-propnanol showed maximum increasing trend against percentage compositions up to 25% composition. This can be explained on the bases of change in structure change, dielectric constant values, ion solvation, crystal forces and ionic radius of the Ca(OH)2. The positive values of the Δ°G explain that the solubility of the Calcium Hydroxide is very low [9]. In other words these Ca(OH)2 when dissolved in these mixed solvent systems followed non-spontaneous dissolution process in forward direction hence, producing positive values of Δ°G [9,26-28]. In the present study the average G° values was obtained in pure water are 28.974 kJ mol-1 and 34.35 kJ mol-1 at 20°C and 70°C are comparable with the reported values 32.3 kJ mol-1 and 43.60 kJ mol-1 at 23°C at 100°C respectively by using volumetric titration method and are 26.79 ± 0.06 at 5 ± 1°C by conductometric method. The slight difference in ΔG° values are due to the selection of temperature because the solubility of calcium hydroxide decreases with the increasing of temperature [26-28].
However the Ca(OH)2 are unfortunately less soluble and has low dissolution in water and mixed solvent system, the dissolution enthalpies of the Ca(OH)2 could not measure directly but have only been evaluate by using equation 4 [1-5,29,30].
The ΔS° values were found to be negative and their change with the percentage composition of mixed solvent systems was almost insignificant and non-linear up to 15% mixed solvent compositions, beyond this up to 25% a slightly decrease in ΔS° values was observed having almost similar values. Since the values of ΔS° were taken in Joule/Mole. Kelvin (J/mol.K) rather then Kilo Joule/ Mole. Kelvin (kJ/ mol.K), therefore the possibility of scattering is more. In present study the average value of ΔS° was found -107.53 Jmol-1K-1 in pure aqueous system while the reported values evaluated in pure water is -150 Jmol-1K-1 and -171 ± 0.43 by simple titration method and conductometric method [13,17]. The significant decrease in the ΔS° values comparative to the reported value is due to the determination of ΔG° at slightly lower temperatures compared to the reported temperature (the present work ΔG° were obtained at 293 ± 1K and 343 ± 1K, while in reported work it was inferred at 298 ± 1K and 373 ± 1K and 277 ± 1K and 303 ± 1K [13,17]. The reason of selection of low temperature in the present work is due to the possibility of evaporation of mixed solvent systems that caused a significant decrease in ΔS° values. Another significant factor for the influence of mixed solvent systems reveals that the mixed solvent systems linearly decrease the ΔS° values compared to pure aqueous system. This behavior justifies that the presence of mixed solvent systems increased orderness in the molecular state of Calcium hydroxide, moreover with 15%-25% compositions such behavior was found to be opposite collectively where a significant decrease in ΔS° values were occurred, justifying the disorderness appeared again or in other words the presence of mixed solvent systems up to 15% compositions decreased the process of association of Calcium hydroxide whereas from 15%-25% compositions it enhanced the process of association of Calcium hydroxide. The values of ΔS° shown in Table 5.
The enthalpy values showed in Table 6, having similar behavior with a non-linearity in ΔH° while plotting against % compositions of mixed solvent systems evaluated by measuring ΔG° at temperatures. The extent of scattering in ΔH° values were more closed having nonlinear trend due to fact the ΔH° values were evaluated in kilo Joule / Mole (kJ/mol.) compared to ΔS° values (when J/mol. K). The ΔH° values were also in negative even in the presence of all these mixed solvent systems similar to the values reported in the literature [13,17]. Obviously it is due to the exothermic nature of calcium hydroxide. A slightly increase in the ΔH° values compared to the literature related to pure aqueous system is due to the selection of temperature [31-35].
Time effect on pH calcium hydroxide solution showed that an increase in pH was observed in different mixed solvent (at different percentage composition of solvent) as compared to blank. It indicates that less dissociation occurred in blank compared to mixed solvent system within 60 minutes. Maximum pH was observed in 2-propanol to 1-proppanol and ethanol while methanol showed intermediate an intermediate behavior. It indicates that maximum dissociation of calcium hydroxide occurred in that solvent which has high pH values as compared to other solvent. For approaching limiting value more time was required in various composition of solvent compared to blank. It showed that less time required to maintain the equilibrium state in blank.