Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2015) Volume 5, Issue 4
Consumer interest in probiotics has dramatically increased in recent years due to improved knowledge of the significant benefits imparted on human health. A specific health issue in which probiotics have been found advantageous is lactose intolerance. Probiotics have demonstrated the ability to act on ingested lactose due to the presence of lactase. The objective of this study was to assess the β-galactosidase (β -gal) activity of commercially available probiotics supplements in the market. Ten supplements were used in this study. Two capsules of each supplement were allowed to activate in MRS broth for 10-12 h. Cultures were then inoculated into TPY broth with lactose (induced) or glucose (uninduced) then incubated at 37°C. After bacterial growth reached the mid log phase (optical density 0.7-0.9; 610 nm), the procedures as outlined by Miller were followed. Activity of β-gal was quantified using the o-nitrophenyl-β-D-galactoside (ONPG) assay. The activity of β-gal in the uninduced group ranged between 0 and 800 Miller unit, whereas there as the induced group ranged from 1 to 1,120 Miller units. When induced, supplement #5 exhibited the strongest enzyme activity at 1,120 Miller units and supplement #10 exhibited the lowest activity. Similarly, supplement #3 exhibited the highest (800 Miller unit) and supplements #1, #2 and #10 did not show any β-gal activity with glucose. These findings indicate that β-gal activity in the ten tested supplements varies. Our results suggest that not all of the commercially available probiotic supplements have the same health benefits.
Keywords: Probiotic supplements, β- galactosidase (β-gal), Health benefits, Lactose intolerance
Bacteria colonize all of the physically available space along the gastrointestinal tract, with varying distribution. These bacteria have invaluable functions in the human body. The relationship between human host and the composition of gut microbiota is primarily mutually beneficial. The metabolic activity of gut microbiota provides the human host with metabolic energy and absorbable substrates and nutrients, while the human host provides the microbiota with a source of energy and nutritious products for growth and development [1]. Health benefits conferred on the host are contingent upon the maintenance of a homeostatic state among the network of the microbiota [2]. However, the microbiota is not indestructible, and the positive attributes provided by the bacteria can be overcome by pathogens and environmental factors, especially after cases of illness and/or medication use
The health conferring microorganisms of probiotics are commercially available to consumers in many forms including probiotic supplements (capsules, gummies, liquids, powders, and tablets). These supplements contain several probiotic bacteria such as Lactobacillus and Bifidobacteria. Probiotics benefit individuals of all ages, and age specific probiotic choices are available. The elderly population is often encouraged to consume probiotics to compensate for the natural decrease of beneficial bacteria in their gut microbiota with increasing age; as well as the increase incident of elderly taking medications [3].
In recent years there has been a dramatic rise in the interest of probiotics due to significant benefits imparted on human health. Scientific evidence supporting the health claims of probiotics stem from in vivo and in vitro studies. Consumer interest in functional foods, including those containing probiotics, has risen in the last 20 years [4]. Despite the predominant presence of probiotic food products available to consumers, probiotic supplements are becoming more popular. A specific health issue in which probiotics have been found advantageous is lactose intolerance. As infants all humans are born with the enzyme lactase (β-galactosidase) which hydrolyses lactose to glucose and galactose in order to be absorbed in the small intestine [5,6]. The prevalence of lactose intolerance varies from population to population. On average, in the United States, 80% of Asian and Native Americans, 75% of African Americans, 51% of Hispanic Americans, and 21% of Caucasian Americans are lactose intolerant [7]. These individuals do not possess the lactase enzyme, and any ingested lactose cannot be digested which leads to the gut microbiota attacking the lactose. Researchers have found the presence of lactase in probiotic products. When the probiotic bacteria reach the intestinal lumen the lactase is lysed by bile, and acts on the lactose that has been ingested. Thus relieving lactose intolerance symptoms [8].
Enzyme activity of probiotic supplements is of interest as a component of the viability of the supplement. In terms of being considered for potential use in alleviating symptoms of lactose intolerance, high β-gal activity is essential. To our knowledge, there are limited studies on this topic. Thus there is a need to determine the presence of β-galactosidase (β-gal) activity in commercial probiotic supplements. We believe this is the first study to determine β-gal activity of commercially available probiotic supplements.
Sample Preparation
Two capsules of each probiotic supplement were added into individual tubes of fresh deMan, Rogosa and Sharpe (MRS) broth respectively, and then mixed for 15 to 30 s using a vortex. Ten probiotic cultures were then incubated for 10-12 h at 37°C for the recovery of the cells. These samples were then used to test β-gal activity of each probiotic supplement
β-gal of probiotic supplements
The activity of β-galactosidase was quantified using the o-nitrophenyl-β-D-galactoside (ONPG) assay as described by Miller [9]. Probiotic supplements were first grown to mid log phase, and an initial O.D. was measured. The cultures were incubated at 37°C until an O.D. of 0.7-0.9 (610 nm) was observed. A 100 μl aliquot of active bacterial cells was washed, and 900 μl of Z buffer (composed of 0.06 M Na2HPO4; 0.04 M NaH2PO4; 0.01 M KCl; 0.001 M MgSO4.7H2O) was added to the pelleted bacterial cells and washed twice. Chloroform was then added at 10 μL per tube and samples were mixed. The mixture was incubated at 37°C on a shaker with open caps for 30 min. After incubation, 200 μl of β-ONPG (4 mg/ml in 0.1 M phosphate buffer) was added to each tube and vortex in order to start the reaction. The reaction was stopped by adding 0.5 mL of 1 N Na2CO3 after the expected yellow color had developed. The time taken for the color to be developed in each tube was recorded. Finally, optical density was recorded at OD420 and OD550. Unit of β-gal produced was calculated using the following equation:
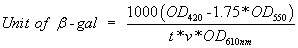
Where t represents time taken for color to develop and v represents volume of the samples. Units of β-gal were calculated as described by Miller [9,10].
β-gal of probiotic supplements after exposure to 3% bile (w/v)
Two capsules of each probiotic supplement was added into individual tubes of fresh MRS broth respectively, and then mixed for 15 to 30 s using a vortex. All supplements were then incubated for 10-12 h at 37°C, for the recovery of the cells. These samples were then exposed to 3% bile (w/v) for 2h at 37°C. The previously mentioned procedure was followed to determine enzyme activity of the samples.
Table 1 shows the β-gal activity of the ten commercial probiotic supplements both in the presence of glucose (uninduced) and lactose (induced). The activity of β-gal in the uninduced group ranged between 0 and 860 Miller unit/mL, and activity in the induced group ranged from 1-1,120 Miller unit/mL. The induction of probiotic supplements with lactose increased the average β- gal activity. Lactose acted as a carbohydrate source on the induction of β-gal activity. Notable increases from uninduced to induced enzyme activity include: supplement #5, 50 to 1,120 Miller units; supplement #6, 28 to 1,068 Miller units; and supplement #8, 26 to 1,065 Miller units. Two supplements exhibited slight increases, but maintained relatively high β-gal activity levels. Supplement #3, 860 to 885 Miller units; and supplement #4, 700 to 715 Miller units. Supplement #5 exhibited the strongest enzyme activity of 1,120 Miller units and supplement #10 exhibited the lowest enzyme activity at 1.45 Miller units. Supplements exhibiting the lowest enzyme activity and lowest increase from uninduced to induce include: supplement #1, 0 to 15 Miller units; supplement #7, 12 to 160 Miller units; and supplement #10, 0 to 1 Miller units.
Initially, ONPG (colorless) is broken down into galactose (colorless) and o-Nitrophenol (yellow). The bright yellow color signifies the breakdown of lactose; increasing intensity of the yellow color indicates increasing β-gal activity (Figures 1a and 1b). Our results were consistent with previous studies in which the presence of lactose in media enhanced β-gal activity when compared to the presence of glucose. We found that the presence of lactose in the growth medium led to β-gal activity enhancement for bifidobacteria and L. acidophilus specifically, [11,12]. We also examined the effect of bile on β–gal activity and found an increase in enzyme activity when bile was included in the medium (Table 2). This finding is consistent with that of Zarate et al. who contributed this enzyme activity enhancement to the permeabilization of probiotic strains by bile. This allowed more substrate to enter the cells to be hydrolyzed by β–galactosidase [13].
Overall lactose intolerant individuals can be well treated by dietary modification and education once properly diagnosed with the condition. Milk and other dairy products can remain in the diet of lactose maldigesters without experiencing symptoms through this dietary modification. This research tested several probiotic supplements containing L. acidophilus, L. reuteri, and L. rhamnosus strains. The high β- gal activity of these supplements (supplements #5, 6, and 8) suggests that consumers should target not only probiotic supplements, but also yogurts, or other forms, containing these strains to possibly mitigate symptoms of lactose intolerance.
We found that β-gal activity may vary among these supplements. This could lead to consumers taking supplements that may not confer expected health benefits. Our research has proven that all probiotic supplements are not equal, specifically in terms of enzyme activity. Previous studies have demonstrated that enzyme activity can be enhanced by the induction of the media with lactose. Therefore, in order to compensate for the variance in the enzyme activity that consumers may unknowingly purchase, it is vital to maintain a balanced and healthy diet. An important component of this diet will be functional foods. Research has shown that functional foods can help enhance the growth and functionality of probiotics in the human microbiota. Future research should investigate how these functional ingredients could induce β-gal activity.
This publication was made possible by grant number NC.X-267-5-12-170-1 from the National Institute of Food and Agriculture and its contents are solely the responsibility of the authors and do not necessarily response the official view of the National Institute of Food and Agriculture.