Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 3
Methylsulfonylmethane (MSM) isan organosulfur phytochemical widely used as a dietary supplement carrying structure/function claims that includethe promotion of joint health. Untargeted metabolomics studies using nuclear magnetic resonance (NMR) have identified MSM in plasma and urine. However, MS-based methodology is more suitable for pharmacokinetic studies designed to explore MSM’s concentration-effect relationship for purposes of establishing dosing guidelines. To address this deficiency, an LC-MS/MS method using MSM, deuterated MSM (MSM-d6, internal standard), and liquid-liquid extraction was developed and validated in accordance with international guidelines. The method proved well-suited for determining MSM levels in human plasma following chronic oral administration (1, 2, or 3 grams daily) for four weeks. The availability of a robust LC-MS/MS method and MSM dose linearity data provides a framework for exploring MSM’s concentration-effect relationship to support various structure/function claims.
Methylsulfonylmethane; LC-MS/MS; Method development
Methylsulfonylmethane (also known as dimethylsulfone, MSM) is a low molecular weight organosulfur compound comprising part of the global sulfur cycle [1,2]. As such, MSMis a ubiquitous dietary ingredient found in numerous foodstuffs including cereal grains, fruits, vegetables, coffee, tea, and cow’s milk [3]. During commercial food processing, dimethylsulfide (DMS), another naturally occurring organosulfur compound, undergoes sequential oxidative reactions to form dimethylsulfoxide (DMSO), which is subsequently converted to MSM [4]. Consequently, significant amounts of MSM also enter the food chain as a by-product of human intervention. In addition to natural and man-made sources, the gut microbiome is speculated to play a contributory role in MSM production via catabolism of dietary methionine to methanethiol, which is a precursor of DMS [5].
Due to its abundance in the food chain, MSM is commonly found in the human metabolome having been detected in various biological matrices including urine, plasma, cerebrospinal fluid, earwax, skin, and sweat [6-15]. MSM’s impact on human health and disease is believed to derive from its ability to act as a sulfur donor, which aids in the synthesis of sulfur-containing amino acids including methionine, cysteine, and taurine. In addition, MSM possesses intrinsic anti-inflammatory and anti-oxidant activity [16]. The pharmacologic properties of MSM coupled with its low potential for human toxicity [17], as evidenced by GRAS (generally recognized as safe) affirmation by the US Food and Drug Administration [18], has led to wide-spread use of MSM as a dietary supplement whose structure/function claims include support of joint health [19-21]. However, establishment of a robust concentration-effect relationship to guide dosing recommendations has proved problematic due to limited sensitivity of published methods in restricted volume samples for detection of MSM.
Various targeted and untargeted bioanalytical methodologies have been used to detect and quantify MSM. The most common technique by far is 1D proton nuclear magnetic resonance (1HNMR), which has been used to quantify MSM in blood and plasma [8,10-12,22,23], urine [6,12], and cerebrospinal fluid [8,14]. The main advantage of NMR over mass spectrometry (MS), viz., the ability to quantify multiple metabolites using a single internal standard, makes NMR an attractive approach in metabolomic studies requiring absolute quantitation. The detection limit for MSM by NMR was estimated to be 2 μM [23]. The detection limit for MSM by MS is similar as NMR. But given the same concentration, MS usually requires much less sample volume than NMR, typically 100 μL used by MS comparing to 500 μL commonly used by NMR. Gas chromatography/mass spectrometry (GC-MS) has been used to quantify MSM in sweat [7], skin [9], and earwax [13]. The inability of GC-MS to recapitulate NMR-based detection of MSM in either urine [6] or cerebrospinal fluid [14], however, calls into question the utility of GC-MS for MSM quantitation across diverse biological matrices. To address the limitations of existing techniques, we developed a sensitive LC-MS/MS-based method for determining MSM plasma levels in humans following chronic oral administration.
Reagents and chemicals
MSM was provided by Bergstrom Nutrition (Vancouver, WA, USA). Deuterated MSM (MSM-d6) was purchased from Sigma- Aldrich (St. Louis, MO, USA). All mobile phase solvents were purchased from Fisher-Scientific (Pittsburgh, PA, USA). Milli-Q water was used for aqueous mobile phase. Pooled normal human plasma was purchased from Innovative Research (Novi, MI, USA).
Human subjects and sample collection
Details of the clinical study are described elsewhere [24]. Briefly, male (n=30) and female (n=15) subjects were randomly assigned to ingest either 1 gram, 2 grams, or 3 grams of MSM daily for four weeks (administered as OptiMSM®; Bergstrom Nutrition; Vancouver, WA). Venous blood samples were collected from an antecubital vein into an EDTA Vacutainer. Blood samples were then centrifuged (15 minutes, 4°C). Plasma was removed and stored at -80°C until analyzed. All study procedures were approved by the University of Memphis Institutional Review Board for Human Subjects Research.
LC-MS/MS parameters
Eight The LC-MS/MS system used for analyte quantification was an Applied Bio systems (AB) Sciex (Framingham, MA) 5500 triple quadrupole mass spectrometer, equipped with a Turboionspray™ (electrospray ionization, ESI) interface. Separation of analytes was carried out using a Poroshell 120 EC-C18 column of 50 × 4.6 mm i.d. and 2.7 μm particle size coupled to a Poroshell 5 × 4.6 mm i.d. UHPLC guard column (Agilent Technologies, Santa Clara, CA). The column was maintained at 35°C using a Shimadzu (Columbia, MD) Nexera XR HPLC system andSIL-20ACXR auto sampler. Mobile phase A (0.1% formic acid in Milli-Q water) and B (0.1% formic acid in methanol) were eluted at 0.5 mL/min. Initial gradient conditions consisting of mobile phase B (10%) were maintained for one minute and then increased linearly to 100% mobile phase B over three minutes. These conditions were maintained for one minute and then decreased to 10% mobile phase B to allow for re-equilibration. Eluent conditions were then returned to initial conditions (10% mobile phase B) over 0.1 minutes and allowed to equilibrate for 0.5 minutes. Total run time was 4.5 minutes with a switching valve directing eluent to MS from one to four minutes.
Positive ionization mode was used for all experiments. Typical ion source parameters included: capillary 3.5 kV, entrance potential (EP) 10 V, collision cell exit potential (CXP) 10 V, declustering potential (DP) 181 V for MSM, 121 V for MSM-d6, collision energy (CE) 35 eV for MSM, 47 eV for MSM-d6, channel electron multiplier (CEM) 1800 V, and a source temperature of 600°C. Dwell time was 150 ms. Curtain gas was set at 20PSI. Both Gas 1(nebulizer gas) and Gas 2 (desolvation gas) were 50 PSI. CAD (collision gas) was 8 PSI. Multiple reaction-monitoring mode (MRM) was used for quantification of MSM (m/z 95.3→65.1) and MSM-d6 (IS, m/z 101.2→65.1). The software program Analyst (Version 1.6.3) was used to process the analytical data.
Preparation of calibration standards and samples
Stock solutions of MSM (1 mg/mL) were prepared in methanol. Stock solution and pooled human plasma were mixed to prepare calibration standards 5, 10, 50, 100, 200, 500, 1000, 2000, 5000, and 10000 μM) and quality control (QC, 5, 250, 1000 μM) samples. Blank samples comprised of blank plasma and IS (1 μg/mL) was prepared using the protein precipitation solution. Prepared standards and samples were stored at -80°C. MSM was extracted from plasma using protein precipitation. Briefly, plasma (50 μL) was added to the precipitation solution (100 μL) comprising 1 μg/mL IS in methanol followed by vortexing (30 seconds) and centrifugation (5 minutes, 4°C, circa 100,000 rpm). The resultant supernatant (25 μL) was diluted in mobile phase B (75 μL) and transferred to a 96-well plate. A sample aliquot (1 μL) was injected for analysis.
LC-MS/MS method validation
Development, qualification, and validation of the method was performed following criteria found in the US Food and Drug Administration (FDA) Guidance for Industry-Bioanalytical Method Validation [25]. Briefly, a plot of the peak area ratio of MSM: MSM-d6 versus the calculated calibration standard concentration was used to construct calibration curves. Calibration curves were constructed for each sample batch from the result of duplicate measurement of seven MSM concentrations. MSM: MSM-d6peak area ratios versus corresponding concentrations were analyzed by linear least-squares regression of the calibration lines to determine slopes (A), intercepts (B), and correlation coefficients. The resultant linear regression equation (1/x weighting) was used to determine the MSM concentration in unknown samples. The lower limit of quantification (LLOQ) was determined over six independent runs using the following criteria of signal to noise>5 and coefficient of variation for accuracy and precision both within 20% for each run. Limit of detection was not evaluated. Chromatographic data were analyzed using the software modules Analyst 1.6.3 and Multi Quant 3.0.2 with manual verification to confirm appropriate peak area integration.
A search for interfering peaks at the retention times of MSM and IS from six blank plasma samples was used to determine method selectivity. The blank spiked plasma sample was injected after the highest calibration sample of MSM for carry-over evaluation. Parallel analytical runs performed either the same day or on three separate days were used to determine intra- and inter-day precision and accuracy, respectively. Chromatographic runs comprised a matrix blank, a set of calibration standards, six replicate LLOQ samples, and a QC sample set. Data were considered acceptable if both precision and accuracy were within ± 15% deviation of the nominal value except as described above for LLOQ determination.
Chromatograms from QC samples (± IS) and blank plasma were compared to evaluate the potential for matrix effect ion suppression. MSM plasma stability was evaluated under various test conditions. Briefly, freeze-thaw sets of QC samples (n=3) stored in plastic vials were allowed to thaw at room temperature and returned to the freezer for 24 hours minimum. The freeze-thaw process was repeated three times. Long-term plasma stability was evaluated following one-week freezer storage (-80°C). In addition, samples were injected six hours apart to assess post-preparative auto sampler stability. Spiked QC plasma samples (n=3) were left on ice for 6 h, then following extraction process to examine bench-top stability. MSM and IS peak areas obtained from freshly prepared samples were used to determine relative stability.
The matrix effect was evaluated by comparing the peaks samples from post-extracted samples at low (5 μM), middle (250 μM) and high (1000 μM) concentrations to those of standard solutions prepared in the mobile phase. The extraction recovery was determined in three replicates by comparing the peak areas of the extracted plasma at 5, 250, 1000 μM pre-extracted with corresponding peaks of plasma samples spiked with MSM post-extraction at the same concentrations.
Method development and qualification
Method development first involved tuning of MS parameters in positive and negative ionization modes for MSM and IS using a 1 μg/mL tuning solution in methanol. For both MSM and IS, the positive ionization mode provided better sensitivity compared to the negative mode. In addition, due to the small mass of MSM (95 g/mol), we evaluated both ESI and atmospheric-pressure chemical ionization (APCI) modes and determined that the positive ESI mode provided the best signal intensity. Consequently, the positive ESI mode was used to further optimize the method.
Optimization of the chromatographic conditions initially focused on identifying the most appropriate extraction solvent between commonly used organic solvents methanol and acetonitrile. Methanol was chosen as it provided a lower background noise than acetonitrile. Moreover, methanol proved superior to acetonitrile in terms of matrix effect, analyte recovery, and peak shape. It was also noted that the inclusion of 0.1% formic acid greatly enhanced peak intensity while gradient elution conditions provided the greatest response intensity, resolution, and peak shape. Finally, protein precipitation (PPT) using methanol (2X volume) was determined to provide the best extraction efficiency of MSM and IS from plasma.
Method validation
Method validation criteria included selectivity, linearity, accuracy, precision, recovery, ion suppression, and stability. Assay selectivity using six separate pooled human plasma lots at the LLOQ was demonstrated by the absence of interfering peaks at the retention times for either MSM or IS in blank extracted plasma. In addition, there were no chromatographic peaks detected in blank pooled human plasma that corresponded to those for either MSM or IS. Typical chromatograms obtained from MSM and IS-spiked pooled human plasma are shown in Figure 1.
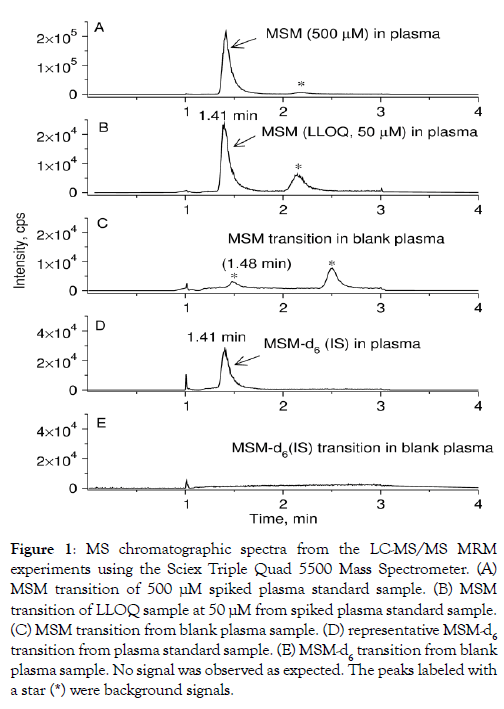
Figure 1: MS chromatographic spectra from the LC-MS/MS MRM experiments using the Sciex Triple Quad 5500 Mass Spectrometer. (A) MSM transition of 500 μM spiked plasma standard sample. (B) MSM transition of LLOQ sample at 50 μM from spiked plasma standard sample. (C) MSM transition from blank plasma sample. (D) representative MSM-d6 transition from plasma standard sample. (E) MSM-d6 transition from blank plasma sample. No signal was observed as expected. The peaks labeled with a star (*) were background signals.
MSM calibration curves demonstrated linearity over a wide range of concentrations 5-5000 M, Figure 2 Assay correlation coefficients (R2) were greater than 0.999 with weighting (1/x). The MSM standard sample with the highest concentration of 10000 μM was also measured, but the accuracy at this highest data point was below 90% due to ion suppression effect and therefore this data point was excluded from the fitting as shown in Figure 2. The average carry-over was 3.4% (below 20%) of the LLOQ for MSM, indicating that the carry-over effect does not affect accuracy and precision, which is permitted by FDA guidelines. MSM LLOQ was found to be 50 μM considering the overlapping interference peak arising from the blank plasma (Figure 2). It turned out that the MSM concentration was greater than 100 μM in all the unknown plasma samples. Using high, medium, and low QC samples, intraday accuracy and precision in plasma ranged from 2.5-5.0% and 3.1-12.5%, respectively, while inter-day accuracy and precision in plasma ranged from 0.1-1.9% and 1.3-13.7%, respectively (Table 1). Using high, medium, and low QC samples, MSM plasma recovery ranged from 89.1-90.5% (Table 1). Matrix effects ranged from 84.2% to 85.2% (Table 1). MSM in plasma was stable at room temperature for at least six hours following precipitation. It was also stable in plasma on ice for six hours before precipitation. MSM degradation was less than 10% following three freeze-thaw cycles (Table 2).
Table 1: Accuracy, precision, and recovery of the method for determination of MSM in plasma.
| Concentration (μM) | Intra-day (n=3) | Inter-day (n=3) | Recovery | Matrix effect | ||
|---|---|---|---|---|---|---|
| Precision (RSD, %) | Accuracy (%) | Precision (RSD, %) | Accuracy (%) | (n=3) | (n=3) | |
| 5 | 12.5 | 5.0 | 13.7 | 1.9 | 89.1 ± 22.5 | 84.2 ± 5.3 |
| 250 | 9.0 | 3.8 | 5.2 | 1.2 | 86.8 ± 13.4 | 82.2 ± 1.2 |
| 1000 | 3.1 | 2.5 | 1.3 | 0.1 | 90.5 ± 7.3 | 85.3 ± 5.9 |
Table 2: Stability results for MSM in human plasma (n=3).
| Stability conditions | Concentration (μM) | RE (%) | RSD (%) |
|---|---|---|---|
| Long-term (1 week, −80°C) | 5 | 2.34 | 15.04 |
| 250 | -0.05 | 3.26 | |
| 1000 | -0.29 | 2.89 | |
| Post-preparative sample (6 h, room temperature) | 5 | 2.02 | 1.05 |
| 250 | -0.20 | 6.26 | |
| 1000 | -0.17 | 10.61 | |
| Pre-preparative sample (6 h, on ice) | 5 | 2.92 | 1.95 |
| 250 | -0.21 | 6.06 | |
| 1000 | -0.18 | 8.81 | |
| Three freeze–thaw cycles (−80°C) | 5 | 3.65 | 31.64 |
| 250 | -0.13 | 8.44 | |
| 1000 | -0.07 | 14.89 |
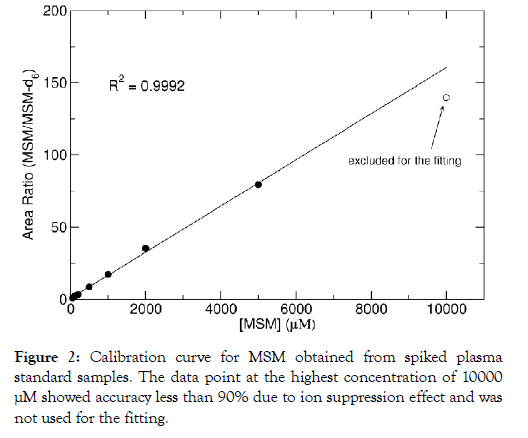
Figure 2: Calibration curve for MSM obtained from spiked plasma standard samples. The data point at the highest concentration of 10000 μM showed accuracy less than 90% due to ion suppression effect and was not used for the fitting.
Pharmacokinetics
An NMR-based bioanalytical method was previously used to determine MSM pharmacokinetics in healthy male subjects following chronic oral administration of MSM (3 grams daily for both two and four weeks) [23-25]. Herein, we expanded our previous study by applying LC-MS/MS to determine MSM pharmacokinetics in healthy male and female subjects receiving orally administered MSM (1, 2, and 3 grams for four weeks). Steady-state MSM concentrations in females were 701.0 ± 202.6, 1455.8 ± 501.8, and 1995.3 ± 1513.8 μM following four weeks of oral MSM administration at 1, 2, and 3 grams, respectively (Table 3). In males receiving the same dosing regimen, steady-state MSM concentrations were 471.5 ± 164.4, 969.3 ± 268.7, and 1841.7 ± 925.7 μM following ingestion of 1, 2, and 3 grams, respectively. No gender-related differences were detected (P>0.05). Analysis of dose proportionality curves indicates a linear relationship between MSM dose and MSM concentration across all three doses with no differences in proportionality (concentration/dose) (P>0.05).
Table 3: Measured MSM levels in human plasma.
| MSM Dose (g/day) | Concentration (μM) | ||
|---|---|---|---|
| All n=45 | Female† n=15 | Male† n=30 | |
| 1 | 542.1 ± 201.1 | 701.0 ± 202.6 | 471.5 ± 164.4 |
| 2 | 1050.4 ± 343.3 | 1455.8 ± 501.8 | 969.3 ± 268.7 |
| 3 | 1892.9 ± 1100.5 | 1995.3 ± 1513.8 | 1841.7 ± 925.7 |
†Genders did not differ significantly
Herein we report the development and validation of the first LCMS/ MS-based bioanalytical method for determining plasma MSM levels following chronic oral MSM administration to humans. Despite the use of a minimal injection (1 μL), the method’s sensitivity was comparable to previous NMR-based methods developed for plasma MSM measurement. The sample volume required in LC-MS/MS process is much less than that using NMR, which could expand the application to small liquid samples. MSM plasma levels were comparable to those achieved previously. Application of the LC-MS/MS method for determination of oral MSM pharmacokinetics in humans revealed two significant findings. First, there appear to be no gender differences in MSM pharmacokinetics. Second, MSM pharmacokinetics are linear over the range of noted when MSM is used by individuals as a dietary supplement. In conclusion, the availability of a robust LC-MS/ MS method and MSM dose linearity data provides a framework for exploring MSM’s concentration-effect relationship to support various structure function claims.
All authors contributed to the study in the following manner: LL, DM, and CRY were responsible for assay development, analysis of blood samples, and MSM pharmacokinetic analysis. RJB and MB were responsible for subject recruitment, screening, and data collection. RJB and CRY were equally responsible for the conception and design of the study, as well as manuscript preparation. LL contributed to data interpretation and manuscript preparation. All authors contributed to and approved of the final manuscript.
Funding for this work was provided by Bergstrom Nutrition and The University of Memphis. In addition, this work was supported by grant (S10OD16226) from the Office of the Director, National Institutes of Health.
CRY and RJB have received research funding from Bergstrom Nutrition, including this study. These contracts paid for direct and indirect costs, as well as salary for CRY. This study was funded by Bergstrom Nutrition, who was consulted in the design of the study. All other authors have no competing interests.
Citation: Lin L, Ma D, Bloomer RJ, Butawan M, Smith WA, Yates CR (2020) Development and application of an LC-MS/MS method for determining methylsulfonylmethane (MSM) levels in human plasma. J Nutr Food Sci 10:773. doi: 10.35248/2155-9600.20.10.773
Received: 15-Apr-2020 Accepted: 04-May-2020 Published: 11-May-2020 , DOI: 10.35248/2155-9600.20.10.1000773
Copyright: © 2020 Lin L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.