Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2015) Volume 6, Issue 1
Objective: Conventional eye drops commonly used in the treatment of glaucoma suffer from short residence time, which results in frequent administration and poor patient compliance. The objective of this work was to develop a liposome-based delivery system for the sustained ocular delivery of latanoprost, a prostaglandin analog commonly used in the management of glaucoma.
Methods: Latanoprost was incorporated into different liposomes that were evaluated using variety of techniques. Selected liposomes were incorporated into different gels and their viscosity and drug release kinetics were evaluated. Optimal liposomal gels were evaluated in vivo in rabbits’ eyes for their irritation potential and ability to reduce intraocular pressure.
Results: Fourier transform infrared and differential scanning calorimetry studies confirmed the interaction between the drug and different excipients in the vesicles, which resulted in drug encapsulation efficiency ≥ 90%. Drug encapsulation efficiency increased with the drug/lipid ratio and encapsulation efficiency ~98% was obtained at drug/lipid ratio of 50%. Vesicles incorporated into Pluronic® F127 gel had sustained drug release where ~45% of the encapsulated drug was released in 2 days. Latanoprost liposomal gels had neither irritation nor toxic effects on the rabbits’ eyes. Further, they had a sustained reduction in the rabbit’s intraocular pressure over a period of 3 days, which was significantly longer than that achieved by the commercial latanoprost eye drops.
Conclusion: These results confirm the potential of latanoprost liposomal gels as viable alternatives to conventional eye drops for the safe and efficient management of glaucoma.
Keywords: Glaucoma; Latanoprost; Liposomes; Ocular delivery; Pluronic; Gel; Thermosensitive
Despite being easy to use, conventional eye drops commonly used in the treatment of primary open-angle glaucoma (POAG) are associated with several disadvantages such as short residence time, variable ocular drug bioavailability and diurnal intraocular pressure (IOP) fluctuation [1]. Further, rapid tear drainage results in rapid drug loss from the eye, which results in a very small percent of the applied dose, ca 5% to successfully penetrate through the cornea [2]. The literature shows a remarkable endeavor to improve the ocular drug bioavailability through overcoming the rapid drug drainage, as well as the transport barrier. To this end, various ophthalmic delivery systems such as suspensions, in situ forming gels, intraocular implants, ophthalmic inserts (mini-tablets), polymeric micelles, dendrimers, liposomes, nanoemulsions, niosomes and contact lenses are under active investigation [3-7].
Liposomes offer a number of advantages as delivery systems for ocular administration, such as improved bioavailability and stability of the incorporated drugs, targeted and controlled release and reduction of drug side effects [8-10]. Their unique structure of a hydrophilic aqueous core surrounded by hydrophobic bilayers allows them to be efficient nanocarriers for both hydrophilic and hydrophobic drugs. Liposomes have excellent biocompatibility due to their phospholipid composition, which resembles the cell membrane structure. However, chemical instability of phospholipids and liposome tendency to degrade, aggregate and fuse are major disadvantages. This can cause premature drug release during storage and after administration [11].
Latanoprost is a lipophilic ester prodrug of prostaglandin F2α with high IOP lowering efficiency. It is a selective agonist of the prostaglandin F prostanoid receptor and exerts its action by increasing uveoscleral outflow rather than altering conventional trabeculo-canalicular aqueous outflow [12,13]. It is well absorbed via the cornea, and is then activated by hydrolysis to the active form latanoprost acid [14,15]. Latanoprost acid experiences a higher penetration resistance through the lipophilic epithelium and the endothelial cells of the corneal membrane [2,15]. Despite the popularity of latanoprost in the management of glaucoma, a careful literature review revealed only handful studies reporting on the development of sustained release delivery systems [1,2,16-19]. Most of these studies used subconjunctival injection of latanoprost liposomes, which prolonged the drug release for up to 120 days. However, subconjunctival injection suffers from major disadvantages, such as the invasive nature of the injection, the need of sterile conditions and the requirement of an ophthalmologist to carry out the injection. This makes this approach inconvenient and patient unfriendly. Further, the repeated subconjunctival injection could lead to ocular infection or other side effects. Thus, an efficient, economical and patient-friendly alternative is highly desirable for the sustained ocular delivery of latanoprost, which we set about to explore.
Herein, we developed and optimized liposomal gels to provide a patient-friendly prolonged ocular delivery of latanoprost, and confirmed their safety and efficacy in reducing the IOP of the rabbits’ eyes. Within this context, latanoprost-loaded liposomes were prepared at different drug/lipid and cholesterol/lipid ratios and their various drug delivery aspects were evaluated. The formulations were characterized using different techniques including differential scanning calorimetry (DSC), fourier-transform infrared spectroscopy (FT-IR), optical microscopy and transmission electron microscopy (TEM). The in vitro drug release kinetics were also evaluated. The effectiveness of optimum latanoprost-loaded liposomal gels in reducing IOP was evaluated in rabbits’ eyes and compared to the commercial Xalatan® eye drops.
Materials
Phosphatidylcholine (phospholipon® 90G) was a gift from Lipoid AG, Switzerland. Latanoprost, cholesterol, hydroxyl propyl methylcellulose (HPMC), Pluronic® F-127 (PL), carbopol 934 (CP) and dialysis membranes (MWCO 12-14 kDa) were purchased from Sigma Aldrich, St. Louis, MO, USA. Chloroform, methanol, diethyl ether, and all other chemicals were obtained from United Company for Chem. Med. Prep., Egypt. All other chemicals were of analytical grade and used as received.
Preparation of latanoprost-loaded liposomes
To determine the effect of preparation method on the drug encapsulation efficiency, latanoprost liposomes were prepared using two different methods, namely thin film hydration and reverse phase evaporation techniques [20-22]. Different liposomal formulations were prepared using varied lipid/cholesterol ratios (Table 1).
| Code | Drug/lipid (wt%) | Compositiona | EE (wt%)b | LC (wt%)c | Particle size (sµm)d |
|---|---|---|---|---|---|
| L-1 | 10 | PC/CH 7:3 | 90.15± 0.58 | 3.64± 0.02 | 1.30 ± 0.24 |
| L-2 | 10 | PC/CH 6:4 | 91.0± 0.24 | 4.41± 0.01 | 0.99 ± 0.08 |
| L-3 | 10 | PC/CH 5:5 | 90.96± 0.07 | 4.63± 0.0 | 1.29 ± 0.46 |
| L-4 | 25 | PC/CH 7:3 | 97.27± 0.07 | 10.83± 0.0 | 1.16 ± 0.52 |
| L-5 | 50 | PC/CH 7:3 | 98.44± 0.01 | 16.39± 0.0 | 1.35± 0.25 |
aPhosphatidyl choline/cholesterol (PC/CH) ratio bPercent encapsulation efficiency, calculated from equation (1), mean of three different formulations ± SD cPercent loading capacity, calculated from equation (2), mean of three different formulations ± SD d Particle size, mean of three different formulations ± SD
Table 1: Composition, encapsulation efficiency (% EE) and loading capacity (% LC) of different liposomes.
For the thin film hydration, known quantities of the drug, cholesterol and phosphatidylcholine were dissolved in a solvent mixture of chloroform and methanol (2:1, v/v) in a 250 ml round bottom flask. The organic solvent was evaporated under reduced pressure at 40°C using rotary evaporator (Büchi, type R 110, Switzerland) resulting in the formation of a thin lipid film on the walls of the flask. To ensure complete removal of the residual organic solvents, the flask was left overnight in a vacuum desiccator. The thin lipid film was hydrated at 37 ± 1°C using Sorensen’s phosphate buffer (pH 7.4). Subsequently, the obtained suspension was vortexed for about 2 min followed by incubation at room temperature for 2-3 h to allow complete hydration of the lipid film. The liposomal suspension was kept in the refrigerator to mature overnight (4°C).
For the reverse phase evaporation technique, the drug, lipid and cholesterol were dissolved in chloroform in a 250 ml round bottom flask followed by formation of thin lipid film as described above. The lipid film was redissolved in a mixture of 10 ml diethyl ether and 10 ml of acetone-water mixture (1:1 v/v). The mixture was sonicated for one minute, swirled by hand and resonicated for another minute. The organic solvents were evaporated on the rotary evaporator at 40°C under reduced pressure for 2 h. The liposomes were allowed to equilibrate at room temperature for 2 h. The liposomal dispersion was kept in the refrigerator to mature overnight at 4°C.
Characterization of latanoprost-loaded vesicles
Determination of drug encapsulation efficiency: To separate the free drug from the encapsulated one, the liposomes were centrifuged at 20,000 rpm at 4ºC for 30 min on a refrigerated centrifuge (Hettich, Germany). The clear supernatant was collected and analyzed by high performance liquid chromatography (HPLC) for drug content after appropriate dilution as described below. The pellet obtained after centrifugation was lysed in methanol and sonicated for 10 min. The concentration of drug in the pellet was determined by HPLC. Percentage drug encapsulation efficiency and loading capacity were calculated as follows:
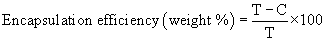 (1)
(1)
Where T is the total amount of drug in the supernatant and sediment, and C is the amount of drug in the supernatant [23].
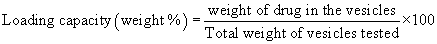 (2)
(2)
Three different formulations were tested and the average ± SD was calculated.
HPLC assay of latanoprost
Concentration of latanoprost was estimated using an HPLC system (Younglin Instrument, Korea) equipped with a vacuum degasser and mixer, a binary pump and UV/Vis detector. The assay was carried out at 25°C using a 7:3 v/v mixture of acetonitrile–water and an Apex ODS C18 5 μm column (4.6 × 250 mm) (Grace Discovery Sciences Inc., USA). The flow rate was maintained at 1.0 mL/min and the injection volume was 20 μL. The drug was monitored by its UV absorbance at 215 nm. The run time was 9 min and the drug had a retention time ~6 min. A stock solution of latanoprost was prepared in acetonitrile and used to construct a calibration curve immediately prior to the assay. The calibration curve was linear in the concentration range of 10 to 50 µg/mL (R2 ≥ 0.999). To determine the latanoprost content of the liposome, a given volume was properly diluted with acetonitrile, filtered through 0.45 µm polytetrafluoroethylene (PTFE) filter and assayed by HPLC. The percent encapsulation efficiency and loading capacity were determined using equations 1-2.
Effect of drug/lipid ratio on the encapsulation efficiency and loading capacity
The liposomes were prepared at drug/lipid ratios ranging from 10-50% w/w and their drug encapsulation efficiency and loading capacity were determined as described above. The liposomes were prepared at lipid/cholesterol ratio of 7:3 (Table 1).
Determination of vesicle size and size distribution
The vesicle size and size distribution were determined using a laser diffraction technique on a Mastersizer X Ver. 2.15 (Malvern instruments Ltd. Malvern, UK). The measurements were performed at 25 °C using a 45-mm focus lens and a beam length of 2.4 mm.
Fourier transform infrared (FT-IR) spectroscopy
FT-IR studies were carried out on individual samples of phosphatidylcholine, cholesterol, latanoprost, physical mixture of excipients and the drug, as well as drug-loaded liposomes. The samples were mixed with IR grade KBr in the ratio of 1:100 and compressed using a hydraulic press under a pressure of 15000 lb. The pellets were scanned in an inert atmosphere over a wave number range of 4000-400 cm-1 in Hitachi 295 spectrophotometer (Hitachi, Tokyo, Japan).
Differential scanning calorimetric (DSC) analysis
Differential scanning calorimetric studies were carried out using differential scanning calorimeter (TA-60, Shimadzu, Japan) for the samples of latanoprost, phosphatidylcholine, cholesterol, physical mixture of the drug with different excipients and latanoprost-loaded liposomes. For this purpose, 5 mg of each sample were thermatically sealed in standard aluminum pans. Each sample was scanned between 30-300°C at a rate of 10°C/min using nitrogen as the purge gas. Indium was sealed in an aluminum pan and used to calibrate the instrument.
Photomicroscopic analysis
Samples of latanoprost-loaded liposomes (L-5) were examined microscopically at magnification of 1000x with a binocular microscope equipped with a camera (Motic, Japan). A drop of liposomal suspension placed on a microscopic slide was examined and photographed.
Transmission electron microscopy (TEM) imaging
The morphology and structure of the same liposomal preparation (L-5) were observed using TEM imaging. The liposomal dispersion was diluted 10-fold using deionized water. A drop of diluted vesicles was applied to a carbon-coated 300 mesh copper grid and left for 1 min to allow some of vesicles to adhere to the carbon substrate. Excess dispersion was removed by a piece of filter paper. The grid was rinsed twice with deionized water for 3-5 S. Next, a drop of 2% aqueous solution of uranyl acetate was applied for 1 S. The remaining solution was removed by absorbing the liquid with the tip of a piece of filter paper and the sample was air-dried. The sample was observed with a transmission electron microscope (JEOL 100 CX, Japan) operated at 80 KV.
Preparation of latanoprost-loaded liposomal gels
Latanoprost-loaded liposomal gels were prepared using different polymers, namely carbopol 934 (CP) 0.5% w/w, Pluronic® F-127 (PL) 20% w/w, 2% w/w and hydroxyl propyl methylcellulose (HPMC) 2% w/w. The required quantity of CP was weighed and dispersed in a small amount of distilled water to prepare an aqueous dispersion. The aqueous dispersion was allowed to hydrate for 4-5 h. The pH was adjusted to 6 by addition of 1% (w/v) triethanolamine solution. PL gels were prepared by the cold method as described by Schmolka [24]. The calculated amount of the polymer was dispersed in cold distilled water and the dispersion was left in a refrigerator at 4°C overnight until the mixture became clear solution. HPMC gel was prepared by dispersing the required quantity of polymer in a small quantity of distilled water. The aqueous dispersion was allowed to hydrate for 4-5 h until clear gel was obtained.
To prepare latanoprost-loaded liposomal gels, selected liposomes (L-5) were prepared as above and centrifuged. The collected pellets were mixed with given quantities of the gels so that the final drug concentration in the gel was 0.005% w/w. Drug-containing gels were vortexed until homogenous gels were obtained. The gels were then briefly sonicated to remove air bubbles.
Viscosity measurement
The viscosity of different gel formulations was determined at room temperature using Brookfield DV+II model LV viscometer. The measurements were made using spindle S-96 at 1.5 rpm.
Drug release studies
In vitro latanoprost release from its liposomal preparations was evaluated using the dialysis bag method. A given weight of drug-loaded liposomal gel (1.0 g, [drug]=3-5 mg/g) was introduced into a dialysis membrane (MWCO 3.5 kDa). The dialysis membranes were transferred to screw-capped glass tubes containing 20 mL of Sorensen’s phosphate buffer pH 7.4 containing 1% v/v Tween®80. The tubes were shaken at 50 RPM in a mechanical water bath shaker maintained at 37°C. Tween®80 was added to maintain perfect sink conditions since latanoprost has a poor aqueous solubility of 40 µg/mL [25]. Corresponding drug-loaded liposomes were prepared as described above and used as a control. At predetermined time intervals, aliquots were taken from the release media and replaced by fresh release medium. The concentration of the drug in the release samples was estimated using the HPLC method described above. The cumulative percent drug released was plotted against time.
Kinetics of drug release
In order to determine the drug release mechanism, in vitro release data were fitted into a Zero-Order (m0–m=Kt), First Order (log m=log m0–Kt/2.303), Higuchi model (m0–m=Kt1/2) and Korsmeyer–Peppas model (m0–m/m0=Ktn) where m is the amount of the drug remaining in the formulation at time t and m0 is the initial amount of the drug in the formulation [26-28]. The regression coefficient values (R2) were calculated for all the models.
Animals: Adult male New Zealand albino rabbits weighing 1.5-2 kg were used in these studies. They were kept in individual cages under standardized conditions of humidity and temperature (temperature: 20–25°C; relative humidity: 40–70%). The rabbits had free access to balanced diet pellets and water with a 12 h-light/12 h-dark cycle. Before the experiments, all eyes were examined with a hand-held slit lamp. Only animals without any signs of ocular inflammation or other observable ocular abnormalities were included in the study. All the experiments were done according to animal ethical guidelines approved by Assiut University, Egypt.
In vivo ocular irritation test: In order to evaluate the potential ocular irritant effect of the tested formulations, the rabbits were divided into two groups, each of 6 rabbits. Group I and II received blank liposomal PL gel and latanoprost-loaded liposomal PL gel, respectively. Latanoprost concentration in the medicated gels was 0.005% w/w. All the glassware used in the experiments was sterilized by autoclaving. The ocular irritancy test was conducted as per the Modified Draize Test [29,30]. Aliquot of 50 µL of the test formulation was instilled into the lower cul-de-sac of the right eye of each rabbit using needleless syringe. The left eye received no treatment and used as a control. The eyelids were gently held together for about 10 S to avoid the loss of instilled preparation. At different time intervals (5, 10, 15, 30 min and 1, 2, 3, 6, 9, 12, 24 h) post instillation each animal was observed for ocular reactions (redness, discharge, conjunctival chemosis, iris and corneal lesions). Ocular irritation of a given formulation was evaluated by assigning a certain score on a scale of 0-4, where 0 means no irritation and 4 means severe irritation [30,31]. The ocular irritation index (Iirr) was calculated by summing up the scores obtained for each criterion at the specified time points. A score of 2 or 3 in any category or Iirr more than 4 was considered as an indicator of clinically significant irritation [31].
In vivo IOP reduction studies: For this test, the rabbits were divided into two groups, each consisting of six rabbits: Group I received latanoprost-loaded liposomes PL gel and Group II received commercial latanoprost eye drops (Xalatan®, Pfizer, Belgium), respectively. Latanoprost concentration in the medicated formulations was 0.005% w/w. Before the measurements, the rabbit eyes were anaesthetized by 1-2 drops of a local anesthetic eye drops (Benoxinate HCl 0.4%, Benox®, EPICO, Egypt). After 1 min, the eyelids were retracted gently with one hand and care was taken to avoid exerting pressure on the eye ball. The IOP was measured using a standardized Schioetz-Tonometer Improved (Gerhard Biro, Germany) while keeping the rabbit eyes directed straight upwards. The tonometer was placed in a vertical position at the center of the cornea. The lower cul-de-sac of right eye of each rabbit of each group (n=6) received 50 µL of the formulation while the contra-lateral eye (left) received no drug and served as a control. Readings in both eyes of each rabbit were taken immediately before drug administration (zero reading) and every hour for a period of 6 h then at 12, 24, 48 and 72 h. All the measurements were done in triplicate by the same investigator. The readings were converted into intraocular pressure using the Eichtabell Table 1955 supplied with the instrument. The ability of different formulations to reduce IOP was expressed as the average difference in IOP (ΔIOP) between the dosed and control eye of the same rabbit using equation (3) [31,32].
ΔIOP=IOP dosed eye–IOP control eye (3)
Statistical analysis
Statistical analysis was carried out using GraphPad Prism software version 5. One-way analysis of variance (ANOVA) was used to analyze the differences between experimental groups. Newman-Keuls method was used as a post-hoc test. A probability of less than 0.05 (p<0.05) was considered statistically significant. All experiments were conducted in triplicate and the results are presented as mean ± SD.
Preparation of liposomes
Vesicle preparation method has an important effect on vesicle lamellarity, drug entrapment efficiency, and particle size. Reverse phase evaporation technique produces large unilameller vesicles with high drug entrapment efficiency, whereas film hydration method produces multilamellar vesicles that are transformed into unilamellar liposomes by sonication. In order to reveal the effect of preparation method on the vesicle properties, formulation L-1 containing PC/CH ratio of 7:3 was prepared by both thin film hydration and reverse phase evaporation techniques and the drug encapsulation efficiency and loading capacity were determined. Liposomes prepared by reverse phase evaporation technique have significantly higher encapsulation efficiency (p<0.05) compared to those prepared by thin lipid film method. Thus, drug encapsulation efficiencies were 90.15 ± 0.58% and 86.01 ± 1.33% for liposomes prepared by reverse phase evaporation and thin lipid film methods, respectively. This finding is in agreement with other studies reporting higher drug entrapment efficiency for vesicles prepared by reverse phase evaporation method compared to ether injection or lipid film hydration methods [33]. Therefore, further preparations in this study were made using reverse phase evaporation technique. All the tested liposomes showed high latanoprost encapsulation efficiency in the range of 88–98% (Table 1). This high drug entrapment efficiency is presumably attributed to the lipophilic nature of the drug which facilitates its partitioning into the lipid bilayers of liposomes [25].
Characterization of latanoprost-loaded vesicles
Effect of cholesterol content on latanoprost encapsulation efficiency: Cholesterol is an important component of vesicles usually added to provide rigidity to the membrane, control permeability and improve plasma stability. It is also known to affect vesicle properties, such as particle size, drug loading capacity, and release rate [34]. Latanoprost vesicles containing 30-50 wt% cholesterol were prepared and their drug loading properties were evaluated (Table 1). Cholesterol content of the liposomes did not affect their drug encapsulation efficiency, which remained at ~90 wt% (Table 1). Increasing the cholesterol content of the liposomes might increase the bilayer hydrophobicity, which in turn decreases the bilayer permeability and prevents further drug entrapment [35]. Latanoprost encapsulation efficiency ~90 weight% was obtained for all the tested formulations confirming the efficiency of the preparation methods.
Effect of drug/lipid ratio on latanoprost encapsulation
Latanoprost-loaded liposomes were prepared at various drug/lipid ratios and their drug encapsulation efficiency and loading capacity were determined. Table 1 shows a general increase in both the drug encapsulation efficiency and loading capacity with the increase in drug/lipid ratio. For instance, increasing the drug/lipid ratio from 10 to 50 wt% resulted in increasing the encapsulation efficiency and loading capacity from ~90 to ~98% and from ~4 to ~16%, respectively. Higher drug loading at higher drug/lipid ratio could be attributed to the availability of high drug concentration during vesicle formation, which favors more drug intercalation into the lipid bilayers. Similar results were previously reported for amphotericin-B loaded liposomes [36]. High drug loading capacity is crucial for successful clinical application of drug delivery systems since it maximizes the drug/excipient ratio and minimizes the harmful effects of unwanted chemicals.
Vesicle size and its distribution
Particle size of vesicles influences the bioavailability of the entrapped drug, as well as the ocular irritation potential of the preparation [37,38]. Particle size of liposomal preparations is shown in Table 1. Liposome size was found to be in the range of 0.99 ± 0.081 to 1.30 ± 0.20 μm with 95% population of the liposomes ≤ 2.0 μm. Log-size distribution curve confirms the normal size distribution of the vesicles with polydispersity index of 0.43.
Shape and morphology of latanoprost liposomes
Transmission electron microscopy images and optical photomicrographs of selected latanoprost liposomes (L-5) are shown in Figure 1. This particular formulation was selected based on their high drug loading capacity which is important for clinical application (Table 1). The photomicrographs reveal the presence of homogeneous unilamellar vesicles with one phospholipid bilayer. The latanoprost-loaded liposomes were spherical in shape, having large internal aqueous core with very little or no aggregation. TEM micrographs confirm that the vesicles have smooth surface and a fairly uniform size. The liposome size obtained from TEM measurement was found to be 0.94 ± 0.22 µm, which is slightly smaller than that obtained by the laser diffraction technique (1.35 ± 0.25 µm) due to liposome drying during sample preparation for TEM measurements. The laser diffraction technique measures the size of liposomes in solution whereas the TEM measures the size of dried liposomes, which is usually smaller than that of the hydrated ones [39].
Fourier-transform infrared (FT-IR) study
The IR spectra of latanoprost, cholesterol, phosphatidylcholine, physical mixtures and drug-loaded liposomes (L-5) are shown in Figure 2. The IR spectrum of pure latanoprost (Figure 2A) shows characteristic peaks at 3374 cm–1 (aliphatic O-H stretching), 2933 cm–1 (aliphatic C-H stretching), 1729 cm–1 (stretching of ester carbonyl group), 1455 cm–1 (O-H bending), 1248 cm–1 (stretching of ester C-O) and 747.89 cm–1 (C-H bending). The IR spectrum of pure cholesterol (Figure 2B) shows characteristics peaks at 3419 cm-1 (aliphatic O-H stretching) and 2934 cm-1 (CH asymmetric stretching of CH3) [40]. The IR spectrum of phosphatidylcholine (Figure 2C) shows characteristic peaks at 2928 cm–1 (C-H stretching), 1749 cm-1 (stretching of ester carbonyl group) and 1245 cm-1 (ester C-O stretching).
The IR spectrum of the physical mixture of latanoprost with cholesterol and lipid (Figure 2D) shows no change in the characteristic bands of the drug or excipients. In contrast, the spectrum of medicated liposomes (L-5) shows minor shift of the drug peaks. For instance, there was a shift for these latanoprost bands: aliphatic alcoholic O-H stretch (3374 to 3365 cm-1), C-H stretching (2933 to 2927 cm-1) and ester C=O stretching (1729 to 1732 cm-1) (Figure 2E). These shifts may be attributed to hydrophobic interactions between latanoprost and liposome constituents. These interactions might be helpful in sustaining the drug release from the liposomes.
Differential scanning calorimetry analysis
DSC analysis was carried out for latanoprost, phosphatidylcholine, cholesterol, their physical mixture and latanoprost-loaded liposomes (L-5) (Figure 3). The DSC thermogram of latanoprost shows an endothermic peak at 153ºC (Figure 3A). The thermogram of cholesterol shows a melting point at 128.60ºC (Figure 3B) whereas that of phosphatidylcholine shows an endothermic peak at 43.66°C, attributed to its phase transition (Figure 3C).
DSC thermogram of latanoprost-loaded liposomes shows disappearance of latanoprost endothermic peak, an increase in the phase transition temperature of phosphatidylcholine from 43.66 to 62.70ºC and a decrease in cholesterol melting point from 128.60 to 99.71°C (Figure 3D). Other studies reported a similar change in the phospholipid transition temperature upon latanoprost incorporation into liposomes [2]. Taken together, DSC results confirm the incorporation of the drug in the lipid bilayers of the vesicles, which could lead to enhanced drug incorporation and sustained drug release.
In vitro latanoprost release
The in vitro release profile of latanoprost from different liposomal gels is shown in Figure 4. Latanoprost has very poor aqueous solubility of 40 µg/mL, therefore 1% v/v Tween®80 was added to the release medium to maintain sink conditions [25]. Low molecular weight non-ionic surfactants such as Tween®80 can be added to release media to maintain sink conditions for hydrophobic drugs [41-43]. Liposomes L-5 were selected for these studies because of their high latanoprost entrapment efficiency (~98 weight%) and loading capacity (~16 weight%) and therefore, high potential for clinical application (Table 1). Latanoprost was slowly released from the liposome suspension; ~40% only was released after 24 h. Incorporation of the liposomes into HPMC and CP gels did not affect the drug release pattern (Figure 4). In contrast, PL gel significantly slowed down the drug release. For instance, after 24 h ~40% of the drug was released from the liposomes, compared to ~30% only for the liposomes dispersed into PL gel. The rate limiting step for the release of hydrophobic drugs from liposome/gel dispersions is the diffusion of the drug through the lipid bilayers rather than through the gel network [44].
This might explain the similar release pattern for the liposome suspension and the liposomes dispersed in CP and HPMC gels. The slower release from the PL gel might be attributed to some specific interactions between the drug and the polymer. Pluronic® F127 is a triblock copolymer of poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide). The central hydrophobic block poly(propylene oxide) might have some hydrophobic interactions with latanoprost which could slow down its diffusion through the gel. Further, PL gel has the highest viscosity among the studied gels, which might retard drug diffusion through the gel and sustain its release (Table 2).
| Gel base | Viscosity (cp)a | |
|---|---|---|
| Liposomal gels | PL (20% w/w) | 16933 ±1200 |
| CP (0.5% w/w) | 1100 ±200 | |
| HPMC (2% w/w) | 4333 ±560 | |
| aMean ± SD, n=3 | ||
Table 2: Viscosity of different latanoprost liposomal gel formulations.
In order to get insights into the mechanism of drug release, the data was fitted according to different release models and the correlation coefficient (R2) and reaction rate constant were calculated. According to the correlation coefficients obtained from fitting the release data into different models, drug release from the liposomes and liposomal gels followed Higuchi diffusion model (Table 3).
| Formula | Zero order | First order | Higuchi model | Korsemeyer–Peppas | ||||
|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | |
| Liposomes (L-5) | 0.9297 | 0.89 | 0.9579 | 0.01 | 0.9815 | 8.05 | 0.9848 | -1.14 |
| Liposomes/CP | 0.9352 | 1.04 | 0.9675 | 0.01 | 0.9840 | 9.37 | 0.9824 | -1.14 |
| Liposomes/HPMC | 0.9184 | 0.93 | 0.9532 | 0.01 | 0.9708 | 8.37 | 0.9708 | -1.19 |
| Liposomes/PL | 0.9824 | 0.87 | 0.9939 | 0.01 | 0.9985 | 7.56 | 0.9958 | -1.51 |
Table 3: Fitting of in vitro release data into different kinetic models.
In vivo ocular irritation studies
The ocular irritation potential of latanoprost liposomal (L-5) PL gels was studied in rabbits since they are available and cheap and have large eyes with specific anatomy and physiology. Further, rabbits eyes are more sensitive to irritation than human eyes [45,46]. All the tested formulations were well tolerated and showed no irritation or toxic effects on the rabbits eyes. The irritation index (Iirr) was zero for all the formulations. This confirms the suitability of these gels for ocular delivery of latanoprost.
In vivo IOP lowering studies
The ability of latanoprost incorporated into liposomal PL gels to lower IOP was tested in normotensive rabbits using the commercially available latanoprost eye drops (Xalatan®) as a control. Liposomes (L-5) suspended into Pluronic® F127 gel were selected for this study. Pluronics undergo thermosensitive transformations from solution to gel at body temperature, which offer the convenience of application as eye drops and increase the ocular residence time of the drug. Figure 5 shows that the two tested formulations rapidly and sharply decreased the IOP with a maximum reduction in the IOP being achieved at 4 h post administration. Subsequently, the IOP of the rabbits treated with Xalatan® started to gradually recover its baseline value and full recovery was obtained at 24 h post administration.
This finding is consistent with the application of Xalatan® eye drops every 24 h in the clinical settings. In contrast, the IOP of the groups treated with latanoprost liposomal (L-5) PL gels returned to its baseline values at much a slower rate and there was a sustained reduction in the IOP over a period of 72 h. Six hours post treatment, the IOP of the rabbits treated with latanoprost liposomal PL gel was consistently and significantly lower than that of the Xalatan®-treated rabbits for the rest of the 72-h measurement period (p<0.05). The maximum reduction in the IOP in all the treated groups was ~8 mmHg, which is consistent with the values reported in other studies for latanoprost and other anti-glaucoma drugs [31,47]. The sustained reduction of IOP achieved by the liposomal gel formulations compared to the drug eye drops might be attributed to the better ocular residence time, enhanced contact with the corneal tissue and sustained drug release [26,48].
Glaucoma is one of the leading causes of irreversible blindness worldwide. In order to overcome the shortcomings associated with conventional anti-glaucoma eye drops, sustained release drug delivery systems need to be developed. We have prepared latanoprost-loaded liposomal gels to sustain its ocular delivery and increase its efficiency. The vesicles were spherical in shape with very little or no aggregation. Control over the drug encapsulation efficiency and loading capacity was achieved by fine-tuning drug/lipid and cholesterol/lipid ratios. Drug encapsulations efficiency ≥ 90% and drug loading capacity ≥ 16% were obtained. Sustained drug release was achieved by incorporation of the drug-loaded vesicles into Pluornic® gels. In vivo studies in rabbits eyes confirmed the potential of these gels as effective ocular delivery system for latanoprost where no irritation potential was recorded. Further, drug-loaded liposomal Pluornic® gels achieved prolonged and superior reduction in the IOP for up to 72 h, which was significantly longer than the commercial latanoprost eye drops. These new formulations could reduce the frequency of drug administration and hence improve patient compliance and the overall therapeutic outcome.