Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2011) Volume 2, Issue 2
HPLC stability-indicating method has been developed for the simultaneous determination of Butamirate citrate and Benzoic acid in pharmaceutical dosage forms. Active compounds were separated on Zorbax SB-C8 column (250 mm × 4.6 mm, 5 μm Agilent) at an ambient temperature. Elution was performed with a mobile phase consisting of acetonitrile (solvent A) and a mixture of 10 gm of sodium lauryl sulphate and 5ml of 1 N sulphuric acid in 1000 ml distilled water (solvent B) at a ratio of 70:30 v/v and a flow-rate of 1.7 ml/min. Detection was performed with UV detector at 205 nm. Cough syrup preparation was subjected to long-term stability in order to demonstrate that storage conditions and degradants from both active compounds did not interfere with the quantification of Butamirate citrate and Benzoic acid. Typical validation characteristics such as linearity, range, precision, accuracy, and selectivity were evaluated for Butamirate citrate and Benzoic acid. Using the novel method, both Butamirate citrate and Benzoic acid were separated successfully. Validation studies demonstrated that the novel method possessed a linear UV response, good system precision and accuracy, high sensitivity and specificity for Butamirate citrate and Benzoic acid.
Keywords: Butamirate citrate; Benzoic acid; HPLC Assay; Longterm Stability; Syrup
Stability testing of pharmaceutical products provides necessary information about the effect of different environmental factors such as temperature, humidity and light on the quality of the finished product, and helps in the determination of the shelf-life as well as the optimal storage and usage conditions [1,2]. It is essential for the analytical procedures used in stability testing of pharmaceutical products to be validated and stability-indicating [1,2]. In addition, the stabilityindicating assay should be able to accurately measure the active ingredients, without interference, from the degradation products, excipients, impurities or other potential process contaminations [3,4].
Butamirate citrate, 2-(2-diethylaminoethyloxy)ethyl 2-phenylbutanoate; 2-hydroxypropane-1,2,3-tricarboxylic acid (Figure 1A), is a non-opioid central cough suppressant which acts on the cough center in the medulla oblongata causing no respiratory suppression, therefore it should be safer than codeine antagonist in cough treatment [5,6]. The drug is mainly prescribed in the form of a syrup containing benzoic acid (Figure 1B) acting as a preservative in order to protect against any microbial growth. Although the formula is widely used in many markets around the world, it is not described officially in any pharmacopeia. There are only few previous studies reporting a method for the determination of butamirate citrate in cough preparations. Those studies mainly focused on the determination of relative bioavailability of the drug after single oral administration [7], the determination of the compound using an optical compensation method [8] or by using derivative spectrophotometric procedures [5]. As there is no method reported in the literature for the simultaneous determination of both butamirate citrate and benzoic acid in cough preparations, it was considered useful to develop and validate a sensitive spectrophotometric method associated with high performance liquid chromatography HPLC. Accordingly, the aim of this work is to adapt the developed method for the quantitation of both compounds in syrup preparations in the presence of their degradation products for application in long-term stability studies of three commercially available batches. Moreover, this study included the follow up of physical characteristics of the cough syrup (pH and specific gravity) as well as the identification and assay of butamirate citrate and benzoic acid.
Materials and Instrumentation
Three batches of Cough Cut syrup containing Butamirate citrate and benzoic acid (Batch numbers: 1618, 512, 3675) were kindly provided by HiPharm for Manufactured Pharmaceuticals (Cairo, Egypt). Butamirate citrate and benzoic acid pharmaceutical standards were obtained from Chemische Werke Hommel GmbH & Co. KG (Lüdinghausen, Germany). Hydrogen peroxide (HPLC grade), sodium hydroxide NaOH (HPLC grade) and hydrochloric acid HCl (HPLC grade) were purchased from Fisher Scientific Co. (Swannee, GA, USA). HPLC grade sodium lauryl sulphate, sulphuric acid and Acetonitrile were obtained from Sigma-Aldrich (ST. Louis, MO, USA) and were used in addition to Milli-Q water in the preparation of HPLC solvents. Millipore syringe filters were obtained from Millipore (Billerica, MA, USA). A 4.6×150 mm reverse phase ZORBAX StableBond SB-C8 HPLC column (5 micron) was purchased from Agilent Technologies (Santa Clara, CA, USA).
The HPLC apparatus (Agilent 1100 series) consisted of a quaternary pump (model G1311A) allowing pulse-free solvent delivery and efficient mixing, an autosampler (G1329A ALS), a preconfigured vacuum degasser (G1322A) and a SHIMADZU UV-1601 UV detector. The apparatus was controlled using Chemstation Software (Agilent).
Stability indicating HPLC method of analysis
A simple and simultaneous method for the determination of butamirate citrate and benzoic was developed in order to be used in the long-term stability study of the cough syrup containing both ingredients.
The chromatographic conditions of the novel method were as follows: the mobile phase was prepared by mixing two solutions, solution A consisting of acetonitrile and solution B prepared in a 1000-ml volumetric flask by mixing 10 gm of sodium lauryl sulphate and 500 ml of distilled water then adding 5ml of 1 N sulphuric acid and completing the volume with distilled water. The two solutions were then mixed such as the ratio of acetonitrile to solution B is 70:30 v/v. The mobile phase was filtered and degassed before use and was delivered at a flow rate of 1.7 ml/min through a C8 column (4.6 x 150 mm, 5 µm Agilent). Both butamirate citrate and benzoic acid were detected at 205 nm.
In order to perform the stability study, the reference solution was prepared by accurately weighing 140.625 mg of butamirate citrate and 112.500 mg of benzoic acid then quantitatively transferring both working standards to a 250-ml volumetric flask, dissolving them in 200 ml mobile phase by sonication and completing to volume with the same solvent to a final concentration of 0.56 mg/ml and 0.45 mg/ml for butamirate citrate and benzoic acid respectively. The test solution was prepared by transferring 37.5 ml sample syrup to a 100-ml volumetric flask followed by completion to volume with the mobile phase to a final concentration of 0.56 mg/ml and 0.45 mg/ml of butamirate citrate and benzoic acid, respectively. Both reference and test solutions were filtered through millipore syringe filter to be ready for injection.
Validation of the novel HPLC method of assay
Stock solutions of butamirate citrate and benzoic acid were accurately prepared with concentrations of 0.84 mg/ml and 0.68 mg/ ml, respectively. Five standard solutions ranging from 0.28 to 0.84 mg/ ml for butamirate citrate and 0.23 to 0.68 mg/ml for benzoic acid were prepared by volumetric dilution of the corresponding stock solutions with mobile phase. A calibration curve for the mean (± SD) butamirate citrate and benzoic acid peak areas against original concentrations was constructed. Linear regression analysis was performed to calculate the regression equation and the limit of detection (LOD) from the equation; LOD = 3.3µ/s; where µ/s is the standard deviation of the y-intercept and s is the slope of the calibration curve.
System precision was determined using standard solutions of 0.28, 0.56 and 0.84 mg/ml for butamirate citrate and 0.23, 0.45 and 0.68 mg/ ml for benzoic acid. Each solution was injected three times and 'within day' precision was calculated as the percent relative standard deviation (RSD%, n=3) of the total peak areas of butamirate citrate or benzoic acid. The same standard solutions were assayed again on the same day for three injections by a different analyst and the analyst-to-analyst ruggedness was calculated as the percent relative standard deviation (RSD%, n=6) of the peak areas of drug substance or benzoic acid. Similarly, inter- day precision was determined after three injections on the next day of the same standard solutions, and calculated as the percent relative standard deviation (RSD%, n=6) of the peak areas due to drug substance or benzoic acid.
The accuracy of the analytical procedure was measured by adding known quantities of butamirate citrate and benzoic acid standards to the placebo syrup containing all excipients of the final product. Accuracy was assessed using 3 samples for each concentration of standard solution covering the specified range -of both butamirate citrate and benzoic acid. The results were then expressed as the percentage difference between the mean measured concentration of both butamirate citrate and benzoic acid and their corresponding nominal values.
The specificity of the HPLC method of assay was determined by subjecting the tested substances to oxidative, alkaline and acidic degradation conditions in order to test the ability of the method to differentiate between the analyte(s) of interest and the degradative products that may be produced under the various degradation processes. The assay was carried out by adding 140.625 mg butamirate citrate and 112.500 mg benzoic acid to hydrogen peroxide (20%), 2N NaOH and 2N HCl in a sealed 10 ml vial and heating in water bath for 20 minutes to test oxidative, alkaline and acidic degradation conditions, respectively. In case of alkaline and acidic degradation, the solution was cooled and neutralized with 2N HCl and 2N NaOH, respectively. The solutions were evaporated just to dryness and the residues were dissolved in 250 ml of the mobile phase then filtered to be ready for HPLC analysis.
Application of the HPLC method of analysis on a long-term Stability study
Long-term stability study: Samples from three different batches of syrup containing butamirate citrate and benzoic acid 7.5 mg and 6.0 mg per 5 ml syrup, respectively, were stored in stability storage cabinet (model ST-5799, Vindonwestech Inc., GA, USA) at a temperature of 30 ± 2°C and relative humidity of 65± 5% for a period of 36 months. The stored samples were examined visually for any changes in color and/ or appearance every month. The stability of the drug product during storage was determined by taking samples at 0, 1, 3, 6, 9, 12, 18, 24 and 36 months intervals and to measure the percent content of butamirate citrate and benzoic acid in cough syrup. The maximum delay time allowed for analysis of long-term stability samples was within 2 to 3 days from the scheduled test date. The peak areas of test samples were compared to those of standard solutions of butamirate citrate and benzoic acid and the percentage of active principal ingredients (%API) remaining was calculated using the following equation:
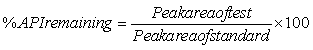
At each time points 3 samples were measured and the results were reported as mean ± SD.
Analysis of physical properties
The effect of storage conditions on the volume, pH and specific gravity of the syrup was measured. The volume of the bottled syrup was measured using calibrated burettes. The limits were set at 97-103 ml. and the reported value was the average of 6 measurements. The pH of the syrup was measured at room temperature using a properly standardized pH-meter, model Accumet XL15 benchtop pH/mV meters (Cole-Parmer, IL, USA). The Limits of pH were 3.5-4.5 and the reported results were the mean values of six measurements. The specific gravity of the syrup was obtained from the weight of a clean, dry and calibrated pycnometer filled with a specific volume of the syrup compared to the weight of similar volume of recently boiled then cooled water contained in it, at room temperature. Limits of specific gravity were set to be 1.16-1.19 gm/ml and the results were mean ± SD (n=6).
Statistical analysis
All mean values were presented as means ± SD. The Student's t-test (two-tailed) was used to evaluate the statistical significance of any differences in mean values in the experimental groups. The ANOVAtest was used to assess the differences in means between treatments. The significance level was set at α = 0.05 for all statistical tests.
System suitability
Working standard solutions of either butamirate citrate or benzoic acid were injected to determine the individual retention time (tR) as well as UV spectrum and the chromatographic purity. Then, working standards mixture solution was injected and the retention time, UV spectrum and chromatographic purity for each substance were confirmed. We considered relative standard deviation (RSD%) for three consecutive injections = 2% [9], resolution between two adjacent peaks = 2 and tailing factor = 2 acceptable values [10]. The mean retention times for benzoic acid and butamirate citrate were 1.88 and 6.10 minutes, respectively (Figure 2). Resolution, perecent relative standard deviation (RSD%) from three replicate injections of working standards mixture solution and tailing factor all lied within the expected ranges. Therefore, system suitability tests confirmed that the chromatographic system was appropriate for the analysis planned to be performed.
Stability indicating HPLC method
The method was validated with respect to parameters including linearity, precision, accuracy, and selectivity and the results are shown in Table 1.
| Parameters | Butamirate citrate | Benzoic acid | |
|---|---|---|---|
| Linearity | slope | 12392 | 23056 |
| intercept | -24.733 | 979.54 | |
| r | 0.9975 | 0.9997 | |
| Range (mg/ml) | 0.28 - 0.84 | 0.23 - 0.68 | |
| Repeatability | RSD% | 0.307 | 0.191 |
| Accuracy | mean recovery | 100.248 | 99.013 |
| RSD% | 0.928 | 0.537 | |
Table 1: Some of the validation parameters for butamirate citrate/benzoic acid assay.
Linearity
The linearity of an analytical procedure is its ability (within a given range) to obtain test results which are directly proportional to the concentration (amount) of the analytes in the sample. Peak areas of active ingredients were plotted versus their concentration and linear regression analysis performed on the resultant curve (Figure 3). The calibration curves constructed for both butamirate citrate and benzoic acid were linear over the concentration range of 0.28 -0.84 mg/ml and 0.23-0.68 mg/ml, respectively. The acceptable correlation coefficient (r) requirement, r> 0.9950 [9] was achieved for both butamirate citrate and benzoic acid showing r values of 0.9975 and 0.9997, respectively. Typically, the regression equations for the calibration curves were found to be y = 12392x + 24.733 for butamirate citrate and y = 23056x + 979.54 for benzoic acid.
Precision
The precision of an analytical procedure expresses the closeness of agreement or the degree of scatter between series of measurements obtained from multiple sampling of the same homogeneous sample under the given conditions. Precision of the assay was investigated with respect to both repeatability and ruggedness. Repeatability was investigated by injecting six replicate samples of three different concentrations of each compound. Results in Table 1 showed that all RSD% were less than 2% for both active ingredients for each of three concentrations tested, which confirms an acceptable degree of repeatability for the used HPLC assay [11]. The ruggedness of the method was assessed by comparison of the intra- and inter-day assay results for butamirate citrate and benzoic acid that has been performed by two analysts. The % RSD values for intra- and inter-day assays of both butamirate citrate and benzoic acid in the cited formulations performed in the same laboratory on different days or by two analysts did not exceed 2%, thus indicating the ruggedness of the method.
Accuracy
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the obtained value [11]. Accuracy of the assay was determined by interpolation of replicate (n = 6) peak areas of three accuracy standards 0.28, 0.56 and 0.84 mg/ml and 0.23, 0.45 and 0.68 mg/ml for butamirate citrate and benzoic acid, respectively, from calibration curves prepared as previously described for each active ingredient. In each case the recovery percent was calculated using the linear regression equation. The results showed that the recovery for all concentration levels for both butamirate citrate and benzoic acid were in the range of 98% - 102%. Accordingly, the analysis method was considered accurate.
Selectivity and stress testing
The results of stress testing studies showed no observed interference for the peaks of degradation products and the principal peaks of both butamirate citrate and benzoic acid. The mean retention times remained at 1.88 and 6.10 minutes for benzoic acid and butamirate citrate, respectively. Therefore, the suggested method of assay showed a high degree of selectivity for butamirate citrate and benzoic acid.
Long-term stability study
As described above, the proposed chromatographic assay allowed a rapid separation and an accurate analysis and quantitation of butamirate citrate and benzoic acid samples. An additional significant advantage of the developed method that is particularly intended for accelerated and long-term stability studies was the one-step procedure for simultaneous analysis of both ingredients. Accordingly, longterm stability samples were used to evaluate the HPLC method. Three different batches of cough syrup containing 7.5 mg and 6.0 mg of butamirate citrate and benzoic acid per 5 ml of syrup, respectively, were exposed to specified zone III long-term stability conditions of 30°C and 65% RH for 36 months [12]. Standard solutions containing 0.56 mg/ml and 0.45 mg/ml of butamirate citrate and benzoic acid, respectively, were prepared in mobile phase and assayed using the HPLC methods. The chromatograms and results of the long-term stability assay are shown in (Figure 4). In all batches, the percentage remaining approximated 100% for both butamirate citrate and benzoic acid formulation content. Statistical analysis of the data showed that there was no significant difference in the percentage remaining for both butamirate citrate and benzoic acid over the period of the study. This indicates that there was no significant degradation by another pathway to additional products that were not detected by the HPLC assay method described in this study. It also provides evidence for the high accuracy of quantification of the tested substances. Similarly, pH and specific gravity measurements (Figure 5) showed that the storage conditions had no significant effect on the physical properties of the syrup.
These results confirm that this novel technique appeared to be sensitive, precise, accurate and selective for studying the long-term stability of butamirate citrate and benzoic acid simultaneously in syrup preparations.
A validated stability-indicating HPLC analytical method has been developed for the simultaneous determination of butamirate citrate and benzoic acid in syrup. The results of stress testing showed that the method is both selective and stability-indicating. The proposed method was also simple, accurate, precise, specific, and had the ability to separate the active ingredients from degradation products and excipients found in liquid dosage forms. The method is suitable for the routine analysis of butamirate citrate and benzoic acid in either bulk powder or in pharmaceutical liquid dosage forms during long-term stability studies. In addition, the HPLC procedure can be applied to the analysis of samples obtained during accelerated stability experiments to predict expiry dates of pharmaceuticals.
The author would like to thank Hi Pharm for Manufactured Pharmaceuticals (Cairo, Egypt), for providing Cough Cut® syrup containing butamirate citrate and benzoic acid for the long-term stability study.