Internal Medicine
Open Access
ISSN: 2165-8048
ISSN: 2165-8048
Research Article - (2022)Volume 12, Issue 1
Quercetin a natural flavanoid has been extensively investigated for its anticancer activity. However, the major disadvantage remained its poor aqueous solubility. In the present research, we investigated the efficacy of folate conjugated quercetin nanoparticles in vitro and further formulated its lotion and studied its skin permeation ability with In vivo activity. The in vitro cellular uptake and antiproliferative potentials of folate conjugated quercetin nanoparticles were evaluated using HaCaT, KB and A431 cell lines. The results showed robust cellular uptake of folate conjugated quercetin nanaoparticles by cell lines. ROS study and Intracellular GSH study revealed that folate conjugated Qu-NPs remarkably reduces the intracellular reactive oxygen species generation and release of GSH. Cells viability studies showed that folate conjugated quercetin nanaoparticless were found to exert profound effect at a concentration of 20 μM on all three cell lines as compared to non-conjugated quercetin nanaoparticles, in a shorter span of time. The skin permeability of the formulation was compared with the marketed drug and was estimated by CLSM study. In vivo studies supported the in vitro activity of folate conjugated quercetin nanoparticles by reducing the epidermal hyperplasia, inflammation and lesion formation.
HaCaT cell lines; KB cell lines; A431 cell lines; Reactive oxygen Species Intracellular GSH; CLSM
Cancer is a progressive cellular disorder. It is characterized by uncontrolled cell growth and proliferation [1]. Various remedies have been developed in due course of time to fight against this disease but, the lack of specificity has still kept the appropriate drug, out of site. Different natural bioactives possess anticancer property and hence are under investigation too [2]. Quercetin, one such polyphenolic flavonoid, has shown several biological effects like anti-inflammatory, anti-cancer, antiproliferative, antimutagenic and apoptosis induction but its use is limited due to its low aqueous solubility [3,4]. The aim of our research was to design and develop such a nano-formulation which can overcome the limitation of poor solubility profile of the drug and can improve cytotoxixity.
In order to achieve this, we have conjugated the nanaoparticless of quercetin with folic acid by applying EDC-NHS chemistry. Folic acid is a vitamin involved in various cellular metabolic pathways. It is an essential element of the nucleotide biosynthesis. Various researches have proved that the entry of folic acid is governed by receptor mediated endocytosis [5].The prominent feature of this process is that the internalization of folate conjugates is free of size specificity that means, they are ideal carriers for small molecules delivery. Further, Folate receptor is a glycopolypeptide membrane anchored protein derivative having high affinity towards folic acid also known as folate binding protein. These are highly expressed on like ovarian, lung, breast, kidney, brain, endometrial and colon cancer cells. This folate receptor on cellular uptake of folic acid undergoes cellular endocytosis by stimulating clathrin independent pathway (polocytosis) which is a characteristic for macromolecule drug delivery [6]. Thus, the major goal of our research was to develop such a delivery system of quercetin that can deliver the drug specifically at the site of action.
For cell culture chemicals
Quercetin was purchased from Sigma Aldrich, USA, Poly (Lactide-co-glycolide) Copolymer (PLGA) was obtained with thanks from Resomer, Evoniks, Germany, Folate, N-(3- dimethylaminopropyl)-N’-Ethylcarbodiimide (EDC) and NHydroxysuccinimide (NHS) was purchased from Sigma-Aldrich, USA and D(+) Mannose was purchased from Himedia. Phosphate buffered saline-PBS (pH5.5 and pH 5.6) used for drug release. All other reagents used were of analytical grade.
Quercetin was purchased from Sigma, USA. All solvents were HPLC grade. Water was purified using a Millipore super Q water system. All the three cell lines were obtained from the National Center for cell science, Pune, India. 3-(4,5- Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma Aldrich Ltd.(India), HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 HAM (DMEM F-12 HAM) with 2 mM Lglutamine supplemented with 10% fetal bovine serum (FBS), 45 IU/ml penicillin and 45 IU/ml streptomycin (HaCaT media) at 37°C in 5% CO2. A commercial kit ThiolTracker™ Violet, Invitrogen, India. For A431 cells were cultured in DMEM supplemented with 10% heat inactivated calf serum, 100 U of penicillin G and 100 U of streptomycin sulfate under 5% CO2 and 95% humidified atmospheric air [7]. All the experiments on animals were also performed after taking approval from Institutional Animal Ethical committee [7].
Synthesis of Qu-NP
Modified Nano precipitation technique was employed to prepare quercetin nanaoparticless. Briefly, 50 mg of PLGA and 20 mg of quercetin was dissolved in acetone and injected in Millipore distilled water and was subjected to sonication with additional volume of water [7]. Then the organic phase was removed by using Rota evaporator (IKA® RB 10) at 37°C under reduced pressure and then it was centrifuged repeatedly at 15000 rpm for 30 minutes and finally lyophilized to obtain dry sample.
Biconjugation with folate
The attachment of folate to the quercetin nanaoparticless was done by applying EDC-NHS conjugation chemistry. For this, the previously suspended quercetin nanaoparticless(1 mg ml-1) in PBS (pH5.6 ) were incubated with EDC in dark for half an hour and then immediately mixed with NHS for at least 6 hours [7,8]. The obtained product is washed with water several times, filtered and mixed with folate at a concentration of 100 μgml-1 in PBS at pH 5.5 for overnight duration. Again, the product is washed with 1 ml PBS, pH 5.5. To remove the excessive reagents and further lyophilized. Finally, the FA-Qu-NPs were redispersed in water [9].
Treatment procedure
HaCaT cells, A431 cells and KB Cells were cultured separately in DMEME F-12 HAM media supplemented with 10% FBS (fetal bovine serum) 100 U of streptomycin and 100 U of penicillin G at 37° C in 5% CO2 Supply. All the cell lines were seeded at a density of 1 × 105 cells/well. After an exposure of 24 hours, the medium was replaced with DMEM containing FAQu- NP ranging from 0-75 μm in concentration [10]. All the samples were prepared in stock solution of DMSO. After the treatment, cells were collected using a hem cytometer. Typhon Blue was dye was used for staining the cells.
Reactive oxygen study
The test was employed to measure the intracellular oxidative stress generated as a result of administration of FA-Qu-NPs, Qu- NPs and Free Quercetin by using 2’,7’-Dichlorofluoresin Diacetate (DCFH-DA) which quantifies intracellular hyperoxides [7,9]. The healthy cells were grown and experiment was carried out in eight sets consisting of eight replicate cells comprising of controls positive and test sample. A 25 μM DCFH-DA in PBS was prepared and all the samples were prepared in this stock solution only. The cells were left under exposure till 24 hours for attachment and then treated with negative, positive control and test sample and incubated for 30, 60, 120, 180, 240 and 300 min. The oxidative stress generated was measured by monitoring the emission at 529 nm of the DCFH-DA.
Cellular uptake study
The cellular uptake of FA-Qu-NPs, Qu-NPs and Free Quercetin were analyzed in HaCaT cells, A431 cells and KB Cells by fluorescence method. Briefly, all the three cell lines were incubated with Hoechst dye for half an hour separately and then were washed thrice with PBS. Then the cells were subjected to Qu –NPs, FA-Qu-NPs and Free Quercetin for different time intervals. Cells were then analyzed under a fluorescence microscope and images were captured using a Nikon Eclipse E600 microscope fitted with a RT spot digital camera [8]. For control readings, cells were incubated with the dye for 30 minutes, washed thrice with PBS and again suspended in medium and further incubated with PBS for half an hour.
Apoptosis study
In order to estimate the apoptotic activity of FA-Qu-NPs, Qu- NPs and Free Quercetin in HaCaT cells, A431 cells and KB Cells, an assay was carried out using Live Dead Assay kit (Invitrogen, India) which works on the activity of intracellular esterase and also specifies the plasma membrane integrity. Briefly, 1 × 105 cells/well were kept under incubation with FAQu- NPs, Qu-NPs and Free Quercetin for 24 hours, then the cells were subjected to dyes Calcein (green fluorescent dye) which is retained by living cells and Ethidium homodimer dye (red fluorescent dye) which enters the damaged cell membranes and get bind to nucleic acid. These two coloured dye’s cells were observed under the fluorescence microscope by counting their numbers on the basis of colour emission.
Intracellular glutathione assay
To estimate the reduction in intracellular glutathione level in HaCaT cells, A431 cells and KB Cells upon FA-Qu-NP, Qu-NP and Free Quercetin exposure, a commercial kit (Thiol Tracker™ Violet) was used. In order to quantify the intracellular glutathione levels in the three cell lines and their respective controls fluroscence microplate reader was employed. The study was carried out in 96 well micro-plates were cells were grown at a density of 1 × 105 cells/well in DMEM with 10% FBS. The cells were left for attachment for 24 hours and then washed twice with fresh PBS (100 μl/well) and then exposed to FA-Qu-NP, Qu-NP and Free Quercetin, in the concentration range of 20-200 mg/ml supplemented with 10% FBS. After the exposure, again the cells were washed with fresh PBS (100 μl/well) and exposed to the commercial dye (Thiol Tracker™ Violet) prepared in PBS at a concentration of 20 μM. The incubatory conditions were maintained at 37°C with 5% CO2 for half an hour [1]. After incubation, the cells were washed thrice to remove the dye with 100 μl/well PBS and the cells were analysed under the reader to measure the wavelength at 405 and 535 nm respectively in Bio-rad micro plate reader.
Preparation of Novel Lotion (NL-4)
The measured quantity of cetyl alcohol, zinc oxide, stearic acid, glycerine and Hydroxyl Propyl Methyl Cellulose (HPMC) were added to luke warm water slowly with constant stirring until a smooth paste is formed. Then it is kept aside for cooling. Lotions were prepared using different concentration of folate conjugated quercetin nanoparticles; free drugs as well as nonconjugated quercetin nanoparticles (2%, 2.5% and 5%).Only optimized products were further preceded for animal studies. Then weighed quantity of folate conjugated quercetin nanoparticles (about 5%) is added and kept under stirring condition for uniform mixing to get the novel targeted lotion (NL-4). In the same way base lotion (NL-1), lotion containing free quercetin (NL-2) and lotion containing non conjugated quercetin nanoparticles (NL-3) were prepared [10].
Physiochemical Characterization
Different types of lotion formulations were developed and the morphology of the nanocargoes present in the formulations were observed by transmission electron microscopy, then all the physicochemical parameters were evaluated to check the safety of the product and along with it in vitro . The pH, thermal stability, fatty content and non-volatile content of the prepared formulations were determined according to the Indian standard guideline. All evaluations were carried out in triplicate presented as mean ± Standard Deviation (SD). All parameters were statistically analysed at 99% confidence level in the column. Analysis of variance ANOVA and Student’s paired t-tests were performed. Differences were considered statistically significant if p<0.01.
Ex-vivo permeation studies
For Ex-vivo permeation study full-thickness goat skin was used for locally fabricated Franz diffusion cell. The skin was clamped between the donor and the receptor chamber of diffusion cell with an effective diffusion area of 2.5 cm. The receptor chamber was filled with freshly prepared phosphate buffer pH 5.5. The diffusion cell was maintained at 32oC and the solution of the receptor chamber was stirred continuously at 350 rpm by using magnetic stirrer with hot plate. The formulation (4.0 g) was gently placed in the donor chamber.
Skin retention study
The skin retention studies of different formulations were performed in order to analyze the content of quercetin in the skin after 24 h of diffusion. At the end of the experiment the skin samples were washed up with water and methanol on both sides and carefully dried. After this procedure a definite amount of methanol was added to each piece of skin. The samples were vortexed for 10 min and stirred overnight. After vortexing, the samples were analyzed by UV spectrophotometer. The result of drug retention studies are reported.
In vivo study in mice
All the experiments on animals were performed after taking approval from Institutional Animal committee. Animals were caged properly and had free access to food and water until use. The animal study was carried out according to the procedures carried out by Kaur et.al with slight modifications. The animals were exposed to UV radiation by the help of a UV lamp (200-400 nm) in a chamber designed specifically in the laboratory to induce photocarcinoma. Anaesthesia was given to the animals before exposing them to radiations to maintain homogenous exposure. The process of irradiation was done with an average irradiance of 3.6 ± 0.4 m W/cm2 to achieve 5 J/cm2 intensity of exposed light radiation for a specific duration of time. The time of light exposure was calculated according to OECD guidelines by following formula. The animals were exposed four times a week for 6 weeks. The dorsal hairs of the mice were removed with a razor and the nude skins were washed with physiological solution.
The animal skin was constantly examined for 6 weeks for the slow progress of photocarcinoma. The evaluation criteria were adoptated from Kaur et.al 2010 with slight modifications. A evaluatory scale was developed according to the skin conditions from 0-5 representation normal skin at 0 and severely damaged skin at 5 scale. Further Skin biopsies were taken every week from each group of animals and was embedded in paraffin after fixing in 10% buffered formalin. Then the sections were sliced with a semi-Automated Rotary Microtome and stained accordingly using Hematoxylin & Eosin (H & E) staining dyes. Finally, the stained specimens were observed under Fluroscence microscope (Nikon®, India), in order to determine the extent of damage caused to skin components.
Characterization of targeted nanosystems
Average size and size distribution of the drug-loaded nanaoparticless were measured by the laser light scattering technique using a particle size analyzer. Samples for measurement were prepared by diluting the material suspension with MilliQ water. Differential Scanning Calorimetry (DSC) is a technique of employed to investigate the melting and recrystallization behavior of crystalline materials. The DSC thermograms of pure Qu, FA, physical mixture. The melting point of pure Qu was found to be 316oC. The DSC thermogram of Qu and FA showed a sharp endothermic peak at 316oC and 250oC respectively. Physical mixture showed two mild peak changes in the position of endothermic peaks. Thus, there was a chemical interaction between Qu and FA. FA-Qu- NPs showed two endothermic peaks in DSC thermogram, one at 250oC for ligand and another at around 155oC for mannitol (cryoprotectant used in lyophilization of FA-Qu-NPs) the peak for PLGA was not visible showing its amorphous nature, the peak of Qu and FA was clearly visible in FA-Qu-NPs. Although, the final peaks of drug and ligand in FA-Qu-PLGA-NPs formulation showed a negative shift towards temperature (Figure 1).
Figure 1: Quercetin Nanaoparticles and folate conjugated quercetin nanopartilces.
Intracellular GSH study
The intracellular GSH measurement reduces significantly when folate conjugated quercetin nanaoparticless was administered, in comparison to the non-conjugated quercetin nanaoparticless and free quercetin. The rate of intracellular GSH depletion was found to be significant at a concentration of 150 mg/l in both A431 and KB cell lines in 24 hours (Figure 2).
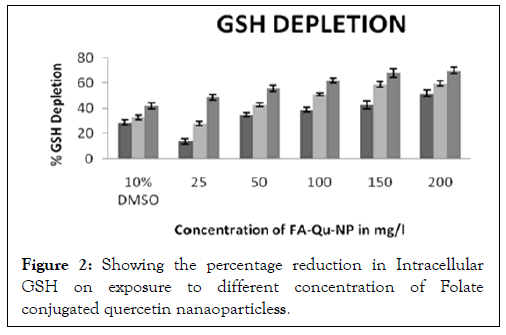
Figure 2: Showing the percentage reduction in Intracellular GSH on exposure to different concentration of Folate conjugated quercetin nanaoparticless.
Reactive oxygen species
The ROS Study has shown that time exposure to folate conjugated quercetin nanaoparticless increases the production of reactive oxygen species generation in all the three cell lines. In case of FA-Qu-NPs, there was tremendous increase in ROS production in all three cell lines. The conjugation of folate with quercetin potentiate the generation of reactive oxygen species leading to cell death.
Apoptosis study
The apoptotic activity was also investigated for FA-Qu-NP, Qu- NP and Free Quercetin in all the three cell lines. In our investigation, we found that the FA-Qu-NP, Qu-NP and Free Quercetin showed a dose dependent apoptotic effect. We finally found that FA-Qu-NP showed a significant apoptotic activity at a concentration below than 20 μM while Qu-NP showed apoptotic activity at a concentration of 30 μM and while that of Free quercetin was observed at a concentration ranging from 45-50 μM.
in vitro cellular uptake study
To corroborate with the above results we also carried out the experiment to investigate the targeting capability of FA-Qu-NP on all the three cell lines using fluroscence microscope. The results of in vitro cellular uptake of FA-Qu-NP, Qu-NP and Free Quercetin were remarkably significant. The cellular uptake response of FA-Qu-NP and Qu-NP was observed relatively slower than free quercetin for early 5 minutes of exposure. The response pattern was relatively similar in HaCaT cells and that of A341 cells while a slightly different cellular uptake pattern was observed for FA-Qu-NP, Qu-NP and Free Quercetin in KB cells. Here, the cellular uptake of FA-Qu-NP started at an initial exposure of 5 minute and lasted for 45 minutes. Moreover, the overall pattern cellular uptake of FA-Qu-NP, Qu-NP and Free Quercetin for all the three cell lines remained in an exponential order i.e. FA-Qu-NP>Qu-NP>Free Quercetin. The reason behind the variations in the cellular uptake response exerted by these cell lines may be the availability of folate receptor at the cellular surface of KB cells which facilitates the cellular uptake of folate conjugated quercetin inside the cell, while in case of A341 cells the role of macrophage and MIF could be anticipated. In HaCaT cell lines no significant changes were observed and it may be due to the lack of folate receptors, on the contrary the role of activated macrophages.
Characterization of novel Lotion
Various parameters like, microscopy, physiochemical evaluation, skin permeation and skin retention studies were carried out in order to characterize the Novel Targeted Lotion (NTL).
Microscopy: The morphological characterization of folate conjugated quercetin nanoparticles incorporated in the lotion was done using transmission electron microscopy at an accelerating voltage of 100 kV (Figure 3). The lotion sample was diluted with distilled water and a drop of the sample was placed on a carbon-coated copper grid to form a thin film and negatively stained by adding a drop of 1% w/v phosphotungstic acid. The grid was allowed to air dry and the samples were viewed and photographed.
Figure 3: The transmission Electron Microscopy (TEM) of Formulated Lotion.
Physicochemical evaluation of lotion: The general physiochemical and psychological parameters were evaluated for the prepared novel formulations. These physicochemical parameters provided information regarding formula stability and skin compatibility. The determination of physicochemical evaluation parameters is essential as they predict the safety as well as stability of the formulations.
Ex-vivo skin permeation studies
Skin penetration study of NL-4 by CLSM: The confocal laser microscopy study was employed to study the skin penetration effect of NL-4 using goat skin in vitro . Goat skin was employed for the purpose of evaluating skin penetrating ability of formulated conjugates because the goat’s skin is anatomically and physiologically similar to that of human skin. The skin penetration of NL-4 was assessed using Rhodamine 123. Briefly, the test samples and the probe containing 0.03% of rhodamine were applied homogeneously and non-occlusively to the skin. The experiments were carried out employing Franz diffusion cells with the receiver chamber filled with phosphate buffer pH 5.5 solutions. After 24 h, the skin was removed and washed with phosphate buffer. The skin was then rapidly frozen by liquid nitrogen and a skin surface perpendicular rectangular piece was taken from the site of drug application with the help of a sharp blade.
Skin retention study
The skin retention studies of different formulations were performed in order to analyze the content of quercetin in the skin after 24 h. The study showed that percentage drug retention of formulations was found higher for FA-Qu-NPs loaded novel lotions (NL-4). The retention was near about similar for Marketed formulation, 0.58 ± 0.30 and that of FAQu- NPs loaded novel lotion 0.78 ± 0.5.
In vivo Study The main objective of our study was to determine the targeting ability of folate conjugated quercetin nanaoparticles (NL-4) dermaly and to evaluate its topical and targeted effect on skin. The macroscopic effect of UV radiation on the animal’s skin was highly distinguishing for evaluation. The non-irradiated skin was free from any type of lesion formation while the irradiated groups developed lesion from the fourth week onwards in nearly 50% of the test animals. At the end of 6 week about 85% of animals developed photocarcinoma lesions (extensive degradation). The cellular components like collagen, elastin, matrix proteins network was found damaged. The animals treated with free quercetin lotion developed lesser skin lesions in comparison to the irradiated group. The animals treated with market formulation, Alkem, India, showed minimal lesion formation at the last week of the irradiation. On discontinuation of the UV irradiations the formulation showed healing effect. The test sample showed nominal skin degradation and negligible lesion formation.
There is a complete loss of epidermal lining and accumulation of elastotic materials with complete degeneration of collagenous meshwork. The generation of epidermal cyst was also observed on continuing the exposure. Denaturation of elastic and collagen fibers in the dermis region. There was Inflammation in the dermal and epidermal region due to cell necrosis. The hair follicles were also found to be damaged histological skin section of the group of animals which were treated with free quercetin containing lotion. This slide is identical in histological characteristics to the histological slide of UV irradiated group. The free quercetin lotion does not exert the desired effect in protecting the skin against radiations shows the effect of marketed formulation on the irradiated animal skin. The skin showed inflammation in deeper layer of the skin with the formation of wrinkles. Although the formulation was found to be very effective in restoring the dermal components of the skin but the skin was found to be rough. The epidermal hyperplasia and dermal elastosis were remarkably reduced in the skin of the animals receiving the application of marketed formulation the animal skins were treated with the test sample (targeted formulation containing folate conjugated quercetin nanoparticles). The result of the application showed no cyst formation and lesser inflammation. The skin was smooth with no sign of wrinkle formation. The collagen and elastin bundle was regular and the hair follicles were also found to be regenerative.
Quercetin is a natural flavanoid exhibiting range of pharmacological activities. It has been proven to be a potent anticancer agent in various studies, despite of several research findings over quercetin against various cancer cells; its mode of action in treating skin cancer is not well addressed. The major issue with quercetin is its polyphenolic nature which reduces its availability due to poor solubility. Hence, we developed folate conjugated quercetin nanaoparticles as targeted drug delivery system and formulated them into a lotion to estimate the targeting ability of these nanocargoes in penetrating skin barrier and reaching the desired site of action. The average particle size was found to be 100.3 ± 7.32 which indicates that the particles were in nanorange. The zeta potential was found to be -23.20 ± 3.5 with a poly dispersity Index less than 1. The entrapment efficiency was found to be 71.48 ± 3.2. Further the SEM analysis confirms the uniform morphology of folate conjugated quercetin nanaoparticless. While the DSC study clearly indicates that the compatibility of FA-Qu-NP. The anticancer potential of folate conjugated quercetin nanaoparticless was evaluated in HaCaT and A431 cell lines due to a close association between keratinosytes and melanocytes while KB cells were used to measure cellular viability because KB cells highly express folate receptor. The results of our study potentiate the role of folate receptor mediate targeting of novel (natural) bioactive for the treatment of skin cancer. The results showed that cellular uptake by the A431 and KB cell lines were higher in comparison to the HaCaT cell lines. While the folate conjugates quercetin nanaoparticless showed an equilibrium effect on all the three cell lines. The reason for this effect may be the migration of the nanocarries across the cellular membrane was estimatedly higher in leaky and ruptured cells in comparison to the normal cells. In a similar study, Zhang et.al synthesized folate mediated poly (3-hydroxy butyrated-CO-3- hydroxyoctonate) nanopartciles of doxorubicin and found that the nanaoparticles were highly effective against HeLa cell lines and showed a remarkable cellular uptake response. Further, they supported their study by evaluating the therapeutic efficacy In vivo too.
In cancer cells, the rate of mutagenicity is greatly governed by the presence or absence of ROS which further leads to DNA damage and chromosomal instability and ultimately causing cancer progression. Secondly, ROS also promote cell survival and proliferation, thus contributing to cancer development. Cancer cells having higher concentration of ROS are more susceptible to quercetin, one of the main dietary flavonoids. Quercetin depletes intracellular glutathione and increases intracellular ROS to a level that can cause cell death. In Reactive Oxygen species generation study, the results revealed that on FAQu- NP exposure, the level of antioxidant present was changed. There was a considerable difference in the ROS level indicating a clear interference of FA-Qu-NP, Qu-NP and Free Quercetin on normal and cancerous cells.
Glutathione is a major component of the intracellular antioxidant defenses. It is present at millimolar concentrations. The GSH system helps in maintaining an appropriate intracellular redox homeostasis. Varios studies have demonstrated that Qu can modify ROS metabolism by directly lowering the intracellular pool of GSH. Taking into consideration the biological and biochemical differences between cancerous and normal cells, the folate conjugated Quercetin nanaoparticless plays a crucial role in internalizing the quercetin inside the cells. The difference in the level of ROS in all the three cell lines can be due to nature of normal, transformed and non-transformed cells. Similarly, in our study we have taken three cell lines in order to comparatively evaluate the binding, internalization and therapeutic efficacy of the nano-conjugates and further confirm their specificity with the targeting moieties. The aim of our study was to enhance the cellular internalization of the conjugate for effective targeting which they formulated a folate bovine serum albumin cis aconitic anhydride doxorubicin prodrug (FA-BSA-CAD) to enhance the specificity of targeting moiety towards a large number of reactive sites. They claimed in their study that folate conjugated DOX showed good therapeutic activity than nonconjugated DOX. In the lieu, Kim et.al also evaluated the targeting ability of 99 mTc labeled FR-PEG conjugate against KB and A549 cell lines. They showed that folate conjugated moiety specifically bounded to the cancer cells irrespective of the normal cells. Finally, the results of the series of experiment draw our attention towards the fact that at a concentration of 100 μg/ml, The FA-Qu-NP exerted significant antiproliferative activity, causing 90% of cell death in 24 hour of incubation. The IC50 values for KB, A431 and HaCaT cell lines were report between 30 to 40 μg/ml indicating a profound dose dependent effect of FA-Qu-NP. It was found that folate conjugated quercetin nanaoparticless exhibited a threefold increase in activity as compared to non- conjugated and free quercetin.
In addition to this, the skin penetrating effect of the targeted novel formulation was studied using CLSM. The result showed that the targeted moieties embedded in the lotion has the ability to penetrate deeper in to the dermal layer and exert its effect. The enhanced penetration effect may be due to the attached folate group which is readily taken up by the cells for their normal cellular functions. The polymeric shell also facilitates its entry into the cells. The percent drug retention of the formulation after 24 hours was satisfactory in comparison to the marketed formulation. Through histological studies, it was found clear that the novel targeted formulation was better than the marketed formulation in restoring the skin vitality as well as in the safe guarding of the skin against UV radiations. The novel lotion was found to significantly improve the collagen and elastin network, decrease inflammation, regenerate hair follicles and decreases the signs of photoaging. The novel formulation was found to be efficient against UV radiation caused skin cancer, not only in reducing oxidative stress in vitro but also as anti-photoaging formulation In vivo . Although extensive studies are left to confirm the overall therapeutic potentials of FA-Qu- NPs conjugates and its formulation, we expect that our study will be small step towards the targeting of natural bioactives in the form of nanoformulations for efficient drug delivery for skin cancer.
The author acknowledges the University Grant Commission- SAP (F. No.3-54/2011(SAP-II)) New Delhi, India, and University Grant Commission (UGC) New Delhi, Under MRP Scheme Major Research project, F. No 39-170/2010 (SR), for financial assistance. Authors also extend their gratitude towards the head of the cosmetic lab, University Institute of Pharmacy, Pt., Ravishankar Shukla University, and Raipur (C.G.) for providing facilities to carry out research work. One of the authors would like to thank Evoniks for providing Polymer (Resomer®) as gift sample to carry out the study.
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[CrossRef] [Google Scholar] [PubMed]
Citation: Gupta A, Kaur CD, Saraf S, Swarnlata S (2022) Development of Novel Folate Conjugated Nanoparticles Based Skin Lotion for Skin Cancer Treatment: in vitro Characterization and In vivo Study. Intern Med. 12:357.
Received: 03-Jan-2022, Manuscript No. IME-21-357; Editor assigned: 05-Jan-2022, Pre QC No. IME-21-357 (PQ); Reviewed: 19-Jan-2022, QC No. IME-21-357; Revised: 24-Jan-2022, Manuscript No. IME-21-357 (R); Published: 31-Jan-2022 , DOI: 10.35248/2165-8048.22.12.357
Copyright: © 2022 Gupta A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.