Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Review Article - (2019)Volume 7, Issue 3
Advancement of monoclonal antibodies (Mabs) to be accepted as a therapy has long history. This article describes the dramatic developmental trend of therapeutic antibody technology and their significant role for treatment of leukemia-lymphoma at the current time. The therapeutic Mab technology development from murine (mouse origin) to fully human resulted in low immunogenicity and high quality Mabs. Chemotherapy is standard care for leukemialymphoma treatments. However, it is associated with high toxicities (side effects), painful and relatively ineffective against certain leukemia-lymphoma subtypes. The remarkable contribution of Von Behring, Paul Ehrlich and the discovery of hybridoma technology marked the beginning of antibodies for therapeutic applications. Rituximab is the first Mab approved by Food and Drug Administration against B-cell malignancies. Today, a number of effective Mabs targeting leukemia and lymphoma were approved and successfully developed. The Mab therapeutic phenomena in the body (also called war in the blood) has various mechanism of cations. In recent years, Mab against leukemialymphoma achieved significantly therapeutic outcomes, eventually this led to traditional chemotherapy treatment free era. We also reviewed that, the therapeutic efficacy of Mabs against leukemia-lymphoma as compared antibody alone and antibody conjugated with potent chemotherapy or cytotoxic drugs. This article extends our understanding of advancements in therapeutic Mabs technology and provides new insights of Mab leukemia-lymphomas therapy. We also encourage further more studies for Mab based diagnostics, treatments of leukemia and lymphomas.
Monoclonal antibody; Leukemia; Lymphoma; Immunotherapy
Mab: Monoclonal Antibody; ADCC: Antibody Dependent Cellular Cytotoxicity; CDC: Complement Dependent Cytotoxicity; ADCP: Antibody Dependent Cellular Phagocytosis; HAMA: Human Anti-Mouse Antibody; BiTE: Bispecific T-cell Engager; ADC: Antibody Drug Conjugates; FDA: Food and Drug Administration; NK cells: Natural Killer Cells; scFV: Single Chain Variable Fragment
Since 1997, FDA’s (Food and Drug Administration) approval of Rituximab anti-CD20; many monoclonal antibodies become as standard drug for leukemia and lymphoma therapies. In recent year, anti‐CC chemokine receptor 4 (CCR4) mogamulizumab monoclonal antibody against adult T‐cell leukemia/lymphoma Therapeutic antibody technology advancement has long history. In terms of antibody history, the remarkable contribution of Von Behring and Paul Ehrlich marked the beginning of the antibody for therapeutic applications. In 1901 Behring was awarded a noble prize for his work on serum therapy against dipteral and tetanus [1].
Paul Ehrlich (also hailed as the founder of chemotherapy) was the person that first proposed side chain theory. He had speculated as the side chain receptors on cells bind to a given pathogen; later on this stated as antigen binding site of the antibody part (peratop) and antibody binding site of the antigen part (epitope). The concept of ‘magic bullets’ was applied to the ground breaking work of Paul Ehrlich, which indicated that fired the specific cell without harming the host cells [2].
In 1975, Kohler and Milstein published an original article in nature journal about continuous cultures of fused cells secreting antibody of predefined specificity [3]. This opened the modern era of antibody research. In the era of modern molecular biology and immunology, monoclonal antibodies are highly applicable as diagnostic tools and provides therapeutic benefit. Monoclonal antibodies have been a standard component of cancer therapy. The first full-length anti-PD-L1 antibody developed by using transgenic rat platform, potentially reduce the risk of immunogenicity and toxicity [4], but there is still much room for improvements.
In recent years, antibodies have had tremendous impacts as research tool in the area of oncology: particularly in the felid of hematological malignancy and stem cell transplantations. The antibody technology application achievement is quite impressive in both therapeutics and diagnostics outcomes. Mab has used in wide range implications such as immune-histochemical (IHC) screening of tumor cell from the normal (cells) tissues (with adequate specificity and affinity potentials) [5] and toxins detection using (ELISA) enzyme linked immune sorbent assay in certain samples [6].
For the past several decades, chemotherapy, radiotherapy and surgical therapy were the most commonly applicable therapies to cure human cancer. However, the side effect of those therapies on cancer patients and the curability successfulness remains a big challenge. Although still at recent chemotherapy is wildly used cancer therapy [7], the high possibility of relapsing, drug resistance and its strong painful nature on cancer patients are considered as a drawback. In the same fashion, radiotherapy killed normal healthy cell while also killing the tumor cells [8] and the surgical therapy has been limited in removing of all the unwanted tumor cells due to the metastasis reason that the cancer disseminated to many body parts. The advancement and rapid growth of the monoclonal antibody technology able to produce an antibody with very high specificity and affinity, which provides big promising hope; monoclonal antibody therapeutics have been approved for over 30 targets and diseases, most commonly cancer [9,10].
In recent year, anti-CC chemokine receptor 4 (CCR4) mogamulizumab monoclonal antibody against adult T-cell leukemia/ lymphoma (ATLL) was chosen as the best therapy; especially when patients have no opportunity to receive hematopoietic stem cell transplantation [11].This showed that Mabs therapies against leukemia-lymphoma has significantly improved.
A literature search of treatment advances for Leukemia-lymphoma based on antibody and chemotherapies was performed for Englishlanguage review articles, using the electronic database of Pubme and google scholars.
The following search terms were input: (Monoclonal antibody, Leukemia, Lymphoma, Immunotherapy) in (Title/Abstract). The authors investigated all relevant studies for quality and publication date and notably, a list of references was added manually.
From murine to fully human
In the last decades of 20 century, the rapid development of monoclonal antibody technology for the therapeutic purpose leads the treatment of various hematological malignances and other cancers [12]. Immunogenicity would have been a big challenge directly to apply the murine antibody to human as a therapy. This is due to the reason that, human anti-mouse antibody (HAMA) response limited the administration of murine antibodies [13].
In a recent perspective, antibody scholars has led to a number of ways to minimize the immunogenicity of non-human antibodies. Humanization of mouse antibody can achieved by minimizing the murine content using the antibody engineering technology. The humanization antibody technology starts primarily through chimeric, humanized, and most recently it achieved fully human therapeutic antibodies [14]. In order to differentiated the antibody type, scientist used a certain given antibody by its type; it can be used their generic suffix name such as –omab (murine antibody), -xmab (chimeric antibody) -zmab (humanized antibody) and -umab for a fully human antibody [15].
Murine antibodies
In 1975 when the hybridoma technology was fully developed, the production of murine antibody became more popular. Murine antibodies are the first antibodies which were applied as therapeutic of human [16]. However, when anti-CD3 murine antibody (OKT3) were accepted as the first antibody therapy for treatment of kidney transplantation due to HAMA (Human anti-mouse antibody) immunogenicity limitation, the antibodies had drawback which limited the antibody efficacy [17]. Despite the fact that murine antibody has limitations for direct human therapeutic application, still they are very useful in vitro and vivo (mouse model) pre-clinical experimental practices. In general, the application of murine antibody as treatment for human was quit limited due to the antibody is a mouse origin and low shelf life of the IgG [18].
Chimeric antibodies
In antibody history, the first human monoclonal antibody was chimeric antibody. In the era of modern molecular biology, the dramatic improvement of protein sequence enabled us to derive chimeric antibody from the murine and human, through protein sequencing [19]. The antibody scholars put a lot of effort in to improving the treatment of antibody for human which results in the development of chimeric and the fully human antibodies [20].
The percentage of chimeric antibody has reached 80-90% human antibody and the remaining is murine type (Figure 1A). Although chimeric antibodies are not fully human, researchers utilized them for diagnostic and showed low immunogenicity as compared to the murine type. The formation of chimeric antibody is based on recombinant DNA technology [19]. The Reopro chimeric antibody has shown significant medical importance in blood clotting in patients through the stimulation of platelets by the receptor proteins [21]. In recent years, scientific studies have shown that anti-CD20 rituximab chimeric antibody is highly applicable as combination therapy of with other antibodies to cure human leukemia and lymphoma [22,23]. However, due to the some percentage of chimeric being of a murine origin, the problem of immunogenicity was still remains unsolved [24].
Humanized antibodies
The interesting high achievement technology of molecular immunology leads to the dramatic change of antibody technology development. The antibody therapeutic improvement was based on the reduction of the murine percentage content [25]. Typically, the humanized antibody is composed of about 90-95% human origin and only the complementary determining region has a murine origin (Figure 1). Humanized antibody derived from murine origin through fusion antibody chain of protein sequence. From its composition point of view, humanized antibodies have great advantage in treatment applications with less immunogenicity [26].
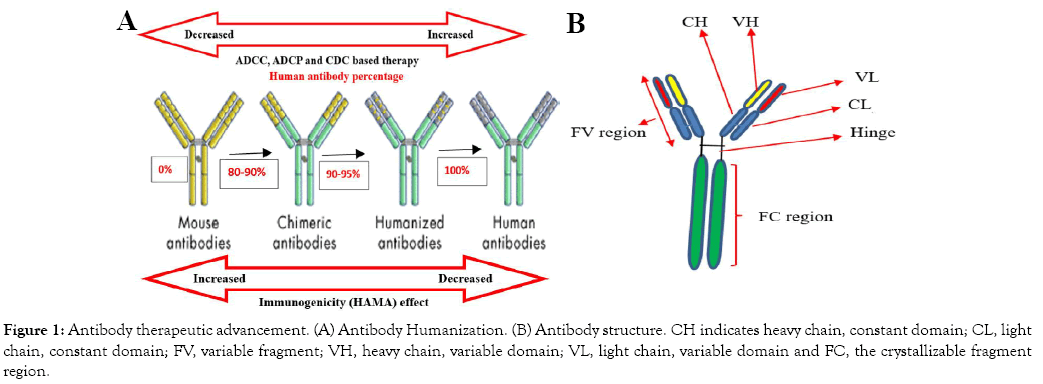
Figure 1: Antibody therapeutic advancement. (A) Antibody Humanization. (B) Antibody structure. CH indicates heavy chain, constant domain; CL, light chain, constant domain; FV, variable fragment; VH, heavy chain, variable domain; VL, light chain, variable domain and FC, the crystallizable fragment region.
In a recent study, humanized anti-CD38 antibody, SG003, using SDR-grafting method possessed stronger antigen binding activity and currently it is in the clinical trial. This antibody enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) in vitro, also enabled inhibitory efficacy of tumor growth in xenograft mice model. A recent study also indicated that humanized antibody is highly applicable for hematological malignancy therapeutics uses [27].
Fully human antibodies
Fully human monoclonal antibodies are the recent antibody generations. These antibodies are highly expected to overcome the problem of the immunogenicity including antibody anti-drug response [28]. For serval decades, antibody scholars were challenged to replace mouse antibody (murine part) completely with human antibody [29]. Surprisingly, advancement in molecular biology has shown incredible rapid antibody technology outcomes and many antibody companies emerged. Nowadays, fully human monoclonal antibodies can be produced using transgenic mice and advanced phage display technologies [30].
The great achievement of fully human monoclonal antibodies using transgenic mice allowed a remarkable opportunities for treatment of several hematological malignances [31]. Recently, fully-human monoclonal antibody anti-CD123, a surface marker which is overexpressed in a variety of hematological disorders, including in an acute myeloid leukemia (AML) enabled kill leukemic cells in vitro ADCC assays, the experiment was done both with cell lines and with patient-derived AML blasts [32].
The Fully human monoclonal antibody ofatumumab was approved for clinical applications in the treatment against B-cell lymphomachronic lymphocytic leukemia cells [33]. The Majority of patients with chronic lymphocytic leukemia (CLL) are older and have prognosis with other cancers. Surprisingly, recently fully human anti-CD20 ofatumumab antibody showed significant therapeutic success at >65 years old aged CLL patients [34].
As it described in Figure 2, in the antibody humanization technology from murine to fully human has improved the immunogenicity problems; furthermore, the antibody-dependent cell-mediated cytotoxicity (ADCC), antibody dependent cellular phagocytosis (ADCP) and complement dependent cytotoxicity (CDC) mechanism of actions increased their therapeutic efficacies. In general, the dramatic rapid therapeutic monoclonal antibody technology from the murine to the fully human provides significant clinical application successes of antibody as a therapy. Furthermore, this outcome affords a significant world economic increment, opened many antibody companies and achieved the best quality of antibodies [35].
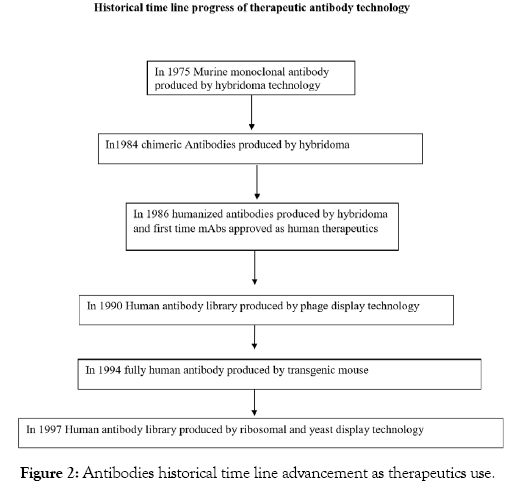
Figure 2: Antibodies historical time line advancement as therapeutics use.
The successful treatment of leukemia and lymphoma has still faces many challenges. Although chemotherapy has been the most widely applicable therapy for hematological malignancies [36], the emerging fields of cancer immunotherapy have shifted to antibody therapies, nowadays a number of monoclonal antibodies enabled cure various leukemia and lymphomas [37].
A recent study indicated that humanized anti-CD20 monoclonal antibody obinutuzumab treatment with venetoclax showed with longer progression-free survival at CLL patients [38]. Venetoclax is a BCL2 inhibitor; it has significant medical importance in (CLL) chronic lymphocytic leukemia patients. Anti-CD20 monoclonal antibody played a great role for B cell malignant. Moreover, combinational therapy of this anti-CD20 and anti-CD37 antibody resulted in significant therapeutic achievements for those B cell malignant patients who are resistance to anti-CD20 monotherapy (Table 1) [39].
| Name | Type | Target | Application |
|---|---|---|---|
| Polatuzumab vedotin | Antibody Drug Conjugate | CD79b | Antibody Drug Conjugate with bendamustine and a rituximab. For adult patients with relapsed or refractory diffuse large B-cell Lymphoma (DLBCL) |
| Rituximab-abbs | Mab | CD20 | For Non-Hodgkin’s Lymphoma (NHL) |
| Pembrolizumab | Mab | PD-1 | For classic Hodgkin's lymphoma (cHL) |
| Brentuximab Vedotin | Antibody Drug Conjugate | CD30 | For adult patients with previously untreated stage III or IV classical Hodgkin lymphoma (cHL) in combination with chemotherapy |
| Moxetumomab Pasudotox-Tdfk | Antibody Drug Conjugate | CD22 | For CD22-directed cytotoxin. Relapsed or refractory hairy cell Leukemia (HCL) |
| Blinatumomab | Mab (Bi-specific T-cell engager) | CD19,CD3 | For B-cell precursor acute lymphoblastic Leukemia (ALL) |
| Gemtuzumab Ozogamicin | Antibody Drug Conjugate | CD33 | For acute myeloid Leukemia (AML) and for relapsed or refractory CD33-positive AML |
| Inotuzumab Ozogamicin | Antibody Drug Conjugate | CD22 | For the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic Leukemia (ALL) |
Table 1: FDA Granted regular approval in recent years, Monoclonal antibodies (Mabs) for lymphoma-leukemia treatments. Data stored since the year of 2017 to 2019 based on FDA approval.
Monoclonal antibody for leukemia treatment
In leukemia antibody therapy, the intense development antibodies targeting CD20, CD19, and CD22 has changed the long-term outcomes. Particularly, in acute lymphoblastic leukemia (ALL) the adults showed quit poor survival as compared with pediatrics (90%) curable rates. Rituximab including inotuzumab ozogamicin and blinatumomab antibodies showed significant medical importance for hematological malignancy treatments [40].
Blinatumomab is FDA approved a bispecific monoclonal antibody. CD3-positive T cells enabled recognize and eliminate CD19-positive acute lymphoblastic leukemia (ALL) blasts. A study reported that treatment with blinatumomab resulted in significantly longer overall survival than chemotherapy. Blinatumomab is also approved for use in patients with relapsed or refractory B-cell precursor ALL [41].
CLL (Chronic lymphocytic leukemia) is one of the common leukemia in elderly patients. Scientific study indicated that deletions of the short arm of chromosome 17 (del(17p)) and/or mutations of the TP53 gene predict resistance to available chemotherapies. For physically fit patients, chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab remains the current standard therapy. For unfit patients, currently available evidence supports two options for a first‐line therapy chlorambucil combined with an anti‐CD20 antibody (obinutuzumab or rituximab or ofatumumab) or a continuous therapy with ibrutinib. If the disease relapses earlier, therapy can be changed using alternative agents such as bendamustine (plus rituximab), alemtuzumab, lenalidomide, ofatumumab, ibrutinib, idelalisib, or venetoclax. Indicated that chemoimmunotherapy is significant and more future studies will be required improving chemoimmunotherapy in CLL patients [42].
A humanized mAb (hUMG1) anti-CD43 showed high reactivity with T-ALL cells. In recently antibody technology, afucosylated of certain antibody showed increased its ADCC/ADCP therapeutic activities. aUMG is afucosylated version of this hUMG1 mAb. Both aUMG1 and hUMG1 antibodies showed significant efficacy on vitro and vivo of T-ALL based therapeutics [43]. Furthermore, the afucosylated version of the antibody was more effective as the result shown on NK-92-CD16+ pre injected NSG (NOD scid gamma) mice [43]. This indicated that, the antibody afucosylate would hopefully the promising choice for leukemia and lymphoma antibody therapies.
For the past 40 years, treatments against acute myeloid leukemia (AML) have been a big challenge. Despite cytarabine and anthracycline being used as stranded care, most patients have poor long-term survival [44]. Anti-CD33 calicheamicin antibody-drug conjugate (ADC), has demonstrated ADCs as a clinically validated option to enhance the effectiveness of induction therapy. Based on mouse xenograft models and in cynomolgus monkeys, the experimental study revealed that a novel anti–CLL-1-ADC, with a highly potent PBD dimer conjugated through a self-immolative disulfide linker enabled depleting of AML tumors and lacks target independent toxicities. This antibody drug conjugate might be effective and safer treatment against AML in humans [44].
In addition to the antibody therapy, some drugs showed significant therapeutic outcomes in hematological malignant patients. Ibrutinib is FDA approved drug that inhibits Bruton’s tyrosine kinase (BTK) which involved in B-cell development and function. Ibrutinib is a targeted therapy has improved the overall survival of patients with chronic graft-versus-host (cGVHD) disease, mantle cell lymphoma (MCL) [45], chronic lymphocytic leukemia/ small lymphocytic lymphoma (CLL/SLL) [46]. Ibrutinib was first approval by FDA as non-chemotherapy combination regimen for treatment-naive patients with CLL/SLL. First, it was approved in Europe and United States; later on China also approved ibrutinib for the treatment of CLL/SLL and mantle cell lymphoma [46]. In the Asia‐Pacific region, a study showed that ibrutinib has resulted in better medical importance as compared with rituximab in relapsed/refractory chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients [46]. However, there is no reported results about antibody with ibrutinib drug combination therapeutic efficacy. Thus, further study will be needed to discover the issue.
For the past over 2 decades, rituximab a chimeric anti-CD20 monoclonal antibody was used for all B-cell malignancies, including diffuse large B-cell lymphoma, follicular lymphoma, and chronic lymphocytic leukemia therapeutic applications [47]. However, its humanized version showed much better in its therapeutic efficacy on various hematological malignancies.
Antibody engineering plays a vital role in the antibody-based therapy of leukemia and lymphoma. In recent years, phage display technology enabled production of monoclonal antibody with high affinity and better therapeutic efficacy. In single chain variable fragment (scFV) small peptide linker connects the VH and VL chain. When an antibody against anti-transferrin receptor-1 (TfR1) was re-engineered from anti-human TfR1 single-chain variable fragment (scFv) antibodies into fully human scFV2-Fcγ1 and IgG1 antibodies; it resulted in a better candidate of antibody therapy for leukiam and lymphoma [48].
Chemoimmunotherapy are recent coming as strands care therapies of acute myeloid leukemia (AML) patients. Elder AML patients (most probably >60 years old) treatment with chemotherapy alone were shown poor prognosis with 5-year survival. A phase 2 study of actinium-225-lintuzumab in older patients with untreated acute myeloid leukemia (AML) revealed that ctinium-225-lintuzumab linked to a humanized anti-CD33 monoclonal antibody achieved as the best therapy for AML elder patients, the study also suggested further study will be required for multiple myeloma patients [49].
100 years ago, the knowledge about the cancer biology was limited and hematological malignancies considered as untreatable diseases. The discovery of DNA structure played a key role for the study of cancer biology; typically, this makes easier to study the role of DNA in the tumor cells. Thus, the outcome of modern molecular biology based scientific study markedly accelerates chemotherapy to become as standard care [50]. Chemotherapy recorded a great achievement on lymphoma and leukemia treatment. However, its extensive toxicity and (failed) relapsing the cases limited chemotherapy utilization. In recent years, the amazing advancement in antibody technology typically antibody engineering enabled to produce therapeutic antibodies including naked antibody, bispecific antibodies and antibody drug conjugates. This leads in to chemotherapy free era treatments for lymphoma and leukemia [50].
Patients at the advanced stage of follicular lymphoma (FL) showed big challenge in terms of relapsing treatments, almost 20% relapsing within two years. Obinutuzumab based treatment resulted in long-term free survival and better achievements against follicular lymphoma [51]. This indicated that Mabs based therapy are more effective than the traditional chemotherapy.
The anti-CD20 chimeric monoclonal antibody rituximab is used as a standard of care for follicular lymphoma. Care patient’s safety including economically and psychologically treatment is significant. Rituximab usually administered intravenously however, recent study at three French teaching hospitals indicated that subcutaneous administration was cost effective (it saved €109.20 per patient per cycle) and favorably chosen by almost all patient compared to intravenous route [52].
It is obvious that collateral effect commonly happened in the treatments of radiotherapy. Radiotherapy has shown damaging of healthy cells while killing the tumor cells. Minimizing the classical radiotherapy effect is important. One of the methods to reduce the side effect of radiotherapy is by delivering a small fraction from the total radiation dose in fraction of time interval with combination of antibody therapy. This allowed normal cells to repair themselves between treatments. The programmed death (PD) pathway is frequently present in the tumor microenvironment and suppresses tumor immunity by inhibiting the activity of tumorinfiltrating lymphocytes particularly, CD8+ lymphocytes. The use of fractionated radiotherapy (RT) with combination anti-αPD-1 monoclonal antibody showed effective in lymphoma combinational therapy [53].
The cell surface expression potential of CD38 in various non- Hodgkin lymphoma(NHL) and multiple myeloma (MM) became an appropriate for immunotherapy [54]. Recently, daratumumab antibody targeted (CD38) showed significant preclinical outcomes in vitro and in vivo models against mantle cell lymphoma, follicular lymphoma and diffuse large B cell lymphoma. The experimental result was evaluated in both antibody alone therapy and with combination chemotherapy. Moreover, surprisingly daratumumab shown FC mediated cytotoxicity, ADCC, CDC and ADCP activity in all subtype of lymphomas. As compared with rituximab, daratumumab showed better results in vivo mouse model based follicular lymphoma therapies [55].
In Japan, mogamulizumab anti-CC chemokine receptor 4 (CCR4) humanized and defucosylated monoclonal antibody was approved for the treatment of relapsed/refractory peripheral T-cell lymphoma, its effectiveness of overall survival was 34% [56]. Another study also evaluated the efficacy of mogamulizumab in 41 pretreated patients with cutaneous T-cell lymphoma [57]. However, the same study conducted in European patients with relapsed or refractory cutaneous lymphoma showed an overall survival (ORR) only11.4 percentage, which did not reach the target ORR of 35% standard set by the protocol, and appeared lower when compared to the previous study of Japanese patients with ORR (34%) [58]. Thus, further more study will be needed to discovery the case.
Naked antibodies, bispecific antibodies and conjugated antibodies
Antibody research is ongoing with many clinical trials to uplift therapeutic qualities. Antibodies such as rituximab (anti-CD20, against hematological malignancy therapy), Avastin (bevacizumab, anti-VEGF or Vascular endothelial growth factor, against lung cancer therapy) were well known. Moreover, Herceptin (trastuzumab, anti-HER2 (receptor human epidermal growth factor receptor 2) against (breast cancer therapy) was widely available in many pharmaceutical antibody companies. Rituximab, Avastin and Herceptin antibodies played significant roles for the past 15 years of antibody cancer therapies [59-61].
Based on their construction origin monoclonal antibody categorized in to three groups: naked antibodies, conjugated antibodies, and bispecific antibodies. Naked antibodies are the antibodies alone without chemotherapy drug or toxin which able to attach specifically to their target antigen and enabled mediated the cell death through effector cells such as natural killer (NK) cells, macrophages and complements [62]. Naked antibodies such as Rituximab, Epratuzumab, Alemtuzumab can induced antibody based cytotoxicity against the target leukemia and lymphomas [63].
Bispecific antibodies are antibodies biologically engineered proteins, which can bind two antigen epitopes at the same time.
In the ordinary monoclonal antibody, the fragrant antigen region occupied by tumor cells. Whereas, in bispecific antibody which makes them different because additionally they have T-cell bispecific engagers. A bispecific antibody has two different light chains and two different heavy chains pair via engineered interchain interactions. Furthermore, bispecific antibodies have high efficacy for cytotoxicity, high affinity and small in size (easily penetrable to tissues) [64,65].
In a recently study, the therapeutic potential of bispecific antibody against CD47 and CD19 showed significant efficacy at elimination of tumor in patients for the treatment of B-Cell Lymphoma and leukemia; for those who were resistance to anti-CD20 targeted therapy. This indicated that bispecific antibody technology might be overcome treatment in hematological malignances resistance to chemotherapy [66]. The co-expression opportunity of CD52 and CD20 in the cell surface B-cell non-Hodgkin lymphoma (B-NHL) and chronic lymphocytic leukemia (CLL) provided benefits to engineer IgG1-like bispecific antibodies. This anti-CD52 and anti- CD20 single chain variable fragment engineered by its fragment constant (FC) region able to cure B-NHL and CLL in patientderived xenograft model [67].
Chimeric Antigen Receptor (CAR) of the effector cell recognizes the target antigen in the tumor cells. The tumor cells of Major Histocompatibility Complex class 1(MHC1) displayed peptide fragments of proteins in to the cytotoxic T cells this enabled to initiate immediate reposes from the immuno system against the certain non-self-antigen. Programmed cell death protein 1(PD-1) is a membrane protein which express in the cell surface and regulates the immunity system. Programmed death-ligand 1(PD-L1) is upregulated on macrophages and dendritic cells. Expression of PDL1 in tumor cells inhibits anti-tumor activists (negatively regulate T cells) PD-1 on the effector cells.
Nowadays, many drug-conjugated antibodies are in the market for leukemia and lymphoma treatments. These antibodies can conjugates with chemotherapies or cancer drugs. The conjugated toxins can bind the cell surface markers and results in tumor cell deaths. Significant effort has led to uplift the treatment potential of monoclonal antibody through modification the naked antibody. This achievement leads to the naked antibodies make more potent, minimizes the use of traditional chemotherapy and improve treatment quality against hematological malignancies. A new investigation about acute myeloid leukemia (AML) reported that the combination of cytarabine with a CD33 targeted antibody drug conjugate (ADC) (containing an indolino-benzodiazepine dimer cytotoxic payload) has shown synergistic effects against leukemia cells both in vitro and in vivo as compared with the ADC alone and cytarabine alone. This indicates the efficacy of antibody with cytotoxic drug conjugate results in better potency against the leukemic cells [68].
Based on vitro and vivo B cell malignancy study report, the novel CD19 targeting antibody drug conjugate hub4-dgn462 showed significant improvements in anti-tumor activity CD19-positive lymphoma-leukemia models .This would provide a best option for the near future of B cell malignancy therapy using antibody drug conjugates [69].
ADCC, ADCP and CDC based therapies
The antibody mechanism of action targeted cancer therapy has various ways: It can be through directly targeting the malignant cells, modifying the host response, delivering cytotoxic moieties and retargeting cellular immunity towards the malignant cells [70].
Recently, therapeutic potential of anti-CD47 (blocking monoclonal antibody) remains a hot issue in hematologic malignancy immunotherapy. Macrophage based ADCP mechanism of action of anti-CD47 for hematologic malignancy therapy is very effective. However, its ADCC based therapy remained controversial yet. The reason is that the mechanism of action for anti-CD47 Mab against the tumor cells is not through direct killing (cytotoxicity) tumors either by NK cells or other effector cell however, it enabled the macrophages to work properly. The combination therapy (synergic therapy) of anti-CD47 and anti-CD20 provides a significant therapeutic application in Non-Hodgkin Lymphoma (NHL) patients [71].
Macrophage based blocking monoclonal antibody showed that “don’t eat me,” signal able to change in to ‘‘eat me’’ signal on cancers that stimulates tumor cell antibody dependent cellular phagocytosis (ADCP) and an anti-tumor T cell response. Anti- CD47 synergizes with rituximab was evaluated in preclinical study. Consequently, anti-CD47 Mab enabled eliminate lymphoma by enhancing Fc receptor-mediated antibody-dependent cellular phagocytosis [72] (Figures 3 and 4).
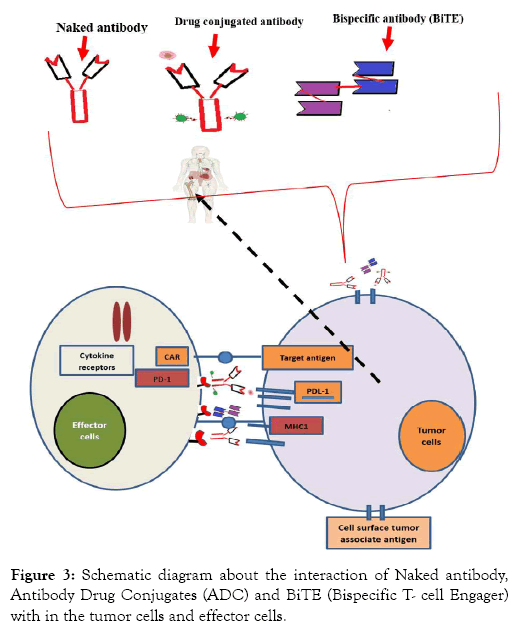
Figure 3: Schematic diagram about the interaction of Naked antibody, Antibody Drug Conjugates (ADC) and BiTE (Bispecific T- cell Engager) with in the tumor cells and effector cells.
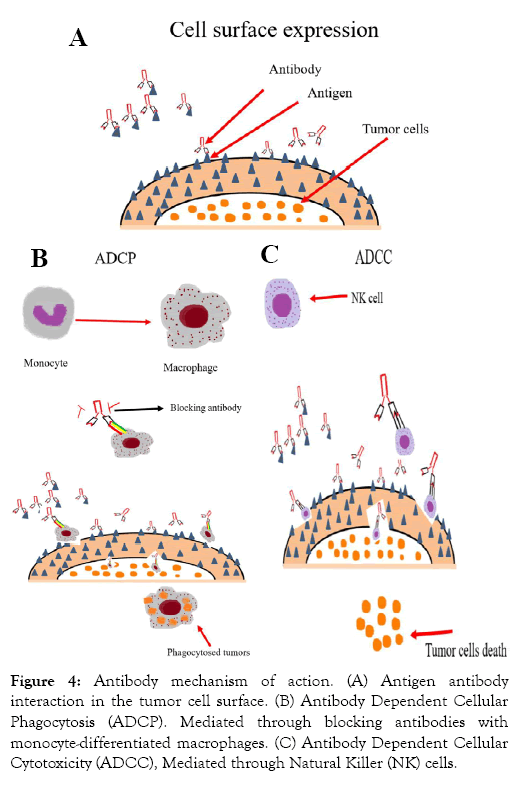
Figure 4: Antibody mechanism of action. (A) Antigen antibody interaction in the tumor cell surface. (B) Antibody Dependent Cellular Phagocytosis (ADCP). Mediated through blocking antibodies with monocyte-differentiated macrophages. (C) Antibody Dependent Cellular Cytotoxicity (ADCC), Mediated through Natural Killer (NK) cells.
Antibody mechanism of action. (A) Antigen antibody interaction in the tumor cell surface. (B) Antibody Dependent Cellular Phagocytosis (ADCP). Mediated through blocking antibodies with monocyte-differentiated macrophages. (C) Antibody Dependent Cellular Cytotoxicity (ADCC), Mediated through Natural Killer (NK) cells.
In the same fashion, as in ADCP mechanism of action, natural killer cells (NK cells), PBMC, (peripheral blood monocytes cells), complements (serum) plays a signifycant roles in antibody-dependent cellular cytotoxicity (ADCC) and Complement dependent cytotoxicity (CDC) therapeutic activities. Recently, mogamulizumab anti-CC chemokine receptor 4 (CCR4) antibody has shown significant ADCC based therapeutic efficacy in treating Adult T cell Lymphoma-Leukemia in Japan [73].
Monoclonal antibodies based treatment was practiced since long time. Nevertheless, the field antibody to get acceptance officially as a therapy passed several challenges and was slow. In the era of modern molecular immunology, the discovery of DNA and protein marked a significant contribution for today’s achievement antibody in diagnostic and therapeutic uses.
After Mabs accepted for leukemia and lymphoma therapy, human treatment immunogenicity would have been a big challenge. In order to solve immunogenicity (Human anti-mouse antibody) (HAMA) effect, monoclonal antibody therapeutic development moved very fast from murine to chimeric, humanized and finally reached fully human. Today, monoclonal antibody can be humanized either from murine origin or can produced human Mab directly using transgenic mice and phage display technologies. Since FDA the first approval of rituximab for B cell malignancy therapy, several antibody companies has emerged. Currently, there are a number of antibodies available for leukemia and lymphoma treatments. Despite chemotherapy was as a standard care in leukemia and lymphoma treatments the relapsing (sometimes failed in therapy) hindered its therapeutic potentials.
In summary, this article addressed the following three major key points. 1. The historical advancement of Mabs from murine to fully human and their significant for therapeutic implications. 2. The therapeutic role of monoclonal antibody in recent perspectives against leukemia and lymphoma. As compared traditional chemotherapy and radiotherapy, Mabs have shown significantly better achievements as promising therapy of leukemia and lymphoma. 3. The basic antibody mechanism of action against leukemia and lymphoma therapy. We also highlighted the therapeutic efficacy of antibody alone, antibody conjugated with cytotoxic drugs and chemotherapies. The idea of war in the blood showed monoclonal antibody utilized differ mechanism of actions to destroy unwanted tumor cells in the body. We suggest that, in vitro and vivo based further experimental study will be needed to identify the effective antibody mechanism of action. We would hope these review provided background scientific evidences and will an asset for further future studied of Mab based leukemialymphoma therapies.
As scientific evidence shown, combinational therapy of monoclonal antibody with potent chemotherapy and cytotoxic drugs resulted in better therapeutic achievements. In the near future, antibody engineering will play very important roles starting from target antigen preparation up to modification of the antibody products. In the coming time, hopefully the clinicians and researchers will put their maximum effort on the production of novel Mabs rather direct use of commercial Mabs; thus, significant discovery will be achieved. In overall, improvement the therapeutic potential of naked antibody, antibody drug conjugates, bispecific antibodies, antibody glycosylation and fucosylation modifications can make Mabs as future promising treatments of leukemia and lymphoma.
This work was supported by National Nature Science Foundation of China (Grant No. 81870135), Fujian Provincial Key Special Projects (Grant No. 2016Y9032, 2016B041, and 2007Y4005), and Construction Project of Fujian Medical Center of Hematology (Gant No.Min201704).
The authors declare no conflicts of interest.
SKY wrote the manuscript. JH revised and approved the manuscript. ML and ZH contributed in revising the manuscript. All authors have read and approved the final manuscript.
Citation: Yirga SK, Lin M, Huang Z, Hu J (2019) Development of Therapeutic Antibody Technology And Recent Perspectives For Leukemia-Lymphoma Treatment. J Leuk 7:259. doi: 10.24105/2329-6917.7.259
Received: 13-Nov-2019 Accepted: 05-Dec-2019 Published: 12-Dec-2019 , DOI: 10.35248/2329-6917.19.7.259
Copyright: © 2019 Yirga SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported by National Nature Science Foundation of China (Grant No. 81870135), Construction Project of Fujian Medical Center of Hematology (Min201704), Special Fund from Fujian Provincial Department of Finance (2016B041), the Cooperation Project of University and Industry in Fujian Province (2017Y4005), Joint Research Project of Health and Education (WKJ2016-2-06), Joint Funds for the innovation of Science and Technology, Fujian Province (2016Y9032).