
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Research Article - (2024)Volume 15, Issue 1
Objective: Evaluating dexmedetomidine's effectiveness in mitigating Postoperative Delirium (POD) and modulating pro-inflammatory markers in elderly patients following thoracolumbar compression fracture surgery.
Methods: This randomized, double-blind, placebo-controlled study was conducted from October 2022 to January 2023 at the Anting hospital in Jiading district, Shanghai. It involved patients aged 65 and above undergoing thoracolumbar compression fracture surgery. Participants were randomized into two groups; the Dexmedetomidine (DEX) group, receiving 0.5 μg/kg/hour of diazepam and the Normal Saline (NS) group. Delirium incidence was assessed on the 1st, 2nd and 3rd days post-surgery using the Confusion Assessment Method (CAM). Levels of Interleukin-1 Beta (IL-1β), Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) were measured pre-operation (T0) and on postoperative day 1 (T1) and day 3 (T3).
Results: In this randomized study of 240 patients, evenly distributed into treatment arms, the administration of dexmedetomidine was significantly associated with a reduced incidence of postoperative delirium. Specifically, 18.2% of patients in the dexmedetomidine group experienced POD, compared to 30.6% in the placebo group (P=0.033). Analysis of cytokine profiles demonstrated a postoperative increase in IL-1β, IL-6 and TNF-α levels followed by a reduction by the third postoperative day (P<0.001 for time-related changes). Notably, IL-6 levels significantly decreased in the dexmedetomidine group during the first postoperative assessment (P<0.001) and TNF-α levels were consistently lower on the first and third postoperative days (P=0.003). No significant difference in IL-1β levels was observed between groups. The occurrence of adverse events was comparable in both cohorts.
Conclusion: Dexmedetomidine significantly reduces postoperative delirium in elderly patients after thoracolumbar compression fracture surgery, primarily on the first day. Additionally, it notably decreases IL-6 and TNF-α levels within the first three days’ post-surgery, highlighting its potential in managing postoperative inflammatory responses in this demographic.
Dexmedetomidine; Elderly patients; Thoracolumbar compression fracture; Pro-inflammatory markers; Postoperative delirium
Postoperative delirium is a common geriatric syndrome characterized by changes in cognition, attention and levels of consciousness following surgery [1]. It is associated with increased morbidity and mortality rates in older patients [2]. The incidence of postoperative delirium varies across different surgical procedures, with rates ranging from 4.1% to 67% in various studies [3,4]. Several risk factors have been identified, including preoperative anxiety, smoking history, hypertension, sleeping pill consumption and open surgical techniques as well as high levels of postoperative pain and opioid use [5-8].
Furthermore, the management of postoperative delirium is crucial, as it can lead to prolonged hospital stays and delayed functional recovery [9]. The development of Post-Operative Delirium (POD) has been associated with an enhanced inflammatory response caused by surgical stress, leading to an increase in pro-inflammatory cytokines in the brain. Found that older adults who develop delirium have significantly elevated levels of pro-inflammatory cytokines in the cerebrospinal fluid and plasm [10]. This supports the notion that surgical stress-induced inflammatory response may contribute to the pathophysiology of POD. Additionally, conducted a longitudinal study and observed an association between plasma cytokine profiles and the development of POD in elderly surgical patients, providing further evidence for the involvement of pro-inflammatory cytokines in POD [11]. Furthermore, demonstrated that electro-acupuncture attenuates surgical pain-induced delirium-like behavior in mice through the remodeling of gut microbiota and dendritic spine, suggesting a potential link between inflammatory modulation and delirium-like behavior [12].
These studies collectively support the hypothesized mechanism of POD involving an enhanced inflammatory response caused by surgical stress and its potential impact on pro-inflammatory cytokines in the brain. The findings underscore the importance of understanding the neurovascular and immune mechanisms underlying postoperative delirium, particularly in the context of aging and surgical interventions. Further research in this area is crucial for developing targeted interventions to mitigate the risk of postoperative delirium and improve perioperative care for vulnerable patient populations.
Dexmedetomidine (DEX), distinguished as a selective α2 receptor agonist, is acclaimed for its sedative, analgesic and anxiolytic efficacy [13]. Functioning as an α2 adrenergic agonist, it orchestrates immune modulation and dampens systemic inflammatory response by curtailing central sympathetic activity [14]. Empirical evidence underscores dexmedetomidine's utility in mitigating postoperative delirium in geriatric cohorts [15,16]. Research elucidating its influence on inflammatory biomarkers, however, remains scant. Preliminary findings suggest dexmedetomidine's mechanism may encompass the suppression of inflammatory pathways, relief from hypoxemia, adept pain management and enhancement of sleep quality [17]. Preclinical investigations have validated its proficiency in repressing central inflammation and reducing serum TNF-α levels in the periphery [18]. Consequently, dexmedetomidine's therapeutic potential in the reduction of postoperative delirium incidence and pro-inflammatory markers in elderly thoracolumbar compression fracture patients warrants further exploration.
This trial aimed to evaluate the effect of intravenously administered dexmedetomidine as an intraoperative sedative on the incidence of postoperative delirium in elderly patients undergoing thoracolumbar compression fracture surgery. To elucidate the relationship between POD and inflammatory response, serum levels of Tumor Necrosis Factor-alpha (TNF-α), Interleukin-1β (IL-1β) and Interleukin-6 (IL-6) were measured at various postoperative time points.
Participants
This study, a forward-looking, randomized, double-blind, placebo-controlled trial was conducted following approval by the Institutional Review Board of Shanghai Jiading District Anting Hospital (Approval: 202207-67). Written informed consent was obtained from all enrolled subjects. The study period spanned from October 2022 to January 2023, targeting elderly individuals undergoing thoracolumbar compression fracture surgery.
Inclusion criteria encompassed: 1) Patients slated for thoracolumbar compression fracture surgical intervention; 2) Age range of 65 to 90 years; 3) American Society of Anesthesiologists (ASA) physical status classification I-III.
Exclusion parameters comprised: 1) Prior psychiatric disorders, chronic psychotropic or analgesic medication usage or a history of alcohol misuse; 2) A Mini-Mental State Examination (MMSE) score of 23 or below, assessed one day prior to surgery (T0); 3) Illiteracy; 4) Auditory or visual impairments or a cerebral vascular event (stroke or transient ischemic attack) within the preceding three months; 5) Severe infection; 6) Communication barriers precluding effective cognitive function assessment.
Participants were randomly assigned to one of two cohorts through a computer-generated sequence; the dexmedetomidine group (n=110) and the Normal Saline (NS) group (n=108). While the anesthesiologist was cognizant of the treatment administered, both the patients and the data collection and analysis team (responsible for the confusion assessment method delirium scale evaluations and POD diagnosis) were blinded to the allocation [19].
Surgical and anesthetic procedures
In this cohort, participants underwent thoracolumbar compression fracture surgeries, including vertebroplasty, kyphoplasty and spinal fusion techniques. These procedures were performed according to well-established standards. The surgical team, consisting of experienced orthopedic surgeons with specialized expertise in spinal surgeries, ensured consistent and precise execution of these complex interventions.
Anesthetic management
The anesthetic protocol for both cohorts was standardized, with the only difference being the use of dexmedetomidine. Continuous monitoring was initiated upon admission to the operating theater, including Electro-Cardiogram (ECG), arterial blood pressure, Heart Rate (HR) and pulse Oxygen Saturation (SpO2). In the prone or lateral position, as appropriate for the surgical approach, ultra-sonographic localization of relevant spinal landmarks was performed. A targeted epidural or spinal anesthesia was administered, using techniques and dosages tailored to the specific type of thoracolumbar surgery being undertaken.
Spinal and general anesthesia techniques
Following nerve block establishment, participants were repositioned after 20 minutes to facilitate spinal anesthesia, elevating the side undergoing surgery. The primary Lumbar (L) puncture site was the L3-4 interspace, where 12 mg of 0.5% ropivacaine was injected into the subarachnoid space. If initial puncture at this site failed, the L2-3 interspace was utilized as an alternate site. In cases where spinal anesthesia was ineffective, a combined spinal-epidural approach was pursued. For situations where lumbar puncture was not viable or combined spinalepidural anesthesia proved unsuccessful, general anesthesia was administered, comprising etomidate (0.3 mg/kg), sufentanil (0.1 mg/kg) and rocuronium (0.6 mg/kg). After intubation, anesthesia maintenance involved controlled ventilation, with a continuous infusion of propofol (100 mg/h) and inhaled sevoflurane.
Dexmedetomidine and normal saline group protocols
In the dexmedetomidine group, dexmedetomidine was intravenously infused at 0.5 mg/kg/h 30 minutes before anesthesia initiation and continued during surgery at a rate of 0.3 mg/kg/h. The normal saline group received a corresponding volume of normal saline. Infusion of both substances was terminated 30 minutes before the end of surgery. The propofol infusion ceased at the conclusion of the surgical process. Postoperatively, for pain management, participants were provided with patient-controlled intravenous analgesia, using a combination of sufentanil and flurbiprofen axetil.
Data acquisition protocol
An exhaustive data acquisition protocol was implemented for all participants, aimed at creating a detailed demographic and clinical dossier. This protocol included a comprehensive range of variables: gender, age, classification according to the American Society of Anesthesiologists (ASA) physical status, Body Mass Index (BMI), educational history, specific characteristics of the thoracolumbar compression fracture, the anesthesia methods applied, intricate details of the surgical procedures, the operative duration and any existing comorbid medical conditions.
Primary outcome assessment
The principal endpoint of this investigation was the rate of Postoperative Delirium (POD), meticulously evaluated on postoperative day 1 (T1), day 2 (T2) and day 3 (T3). Preliminary evaluation for each participant was conducted using the Richmond Agitation-Sedation Scale (RASS) [20]. Those registering a RASS score higher than 4 were further assessed with the Confusion Assessment Method (CAM) scale to determine the presence of POD. The CAM diagnostic criteria encompass: (1) acute onset with fluctuating course, (2) inattention, (3) disorganized thinking and (4) altered level of consciousness. A positive CAM finding required the concurrent presence of criteria 1 and 2, in addition to either criterion 3 or 4.
Moreover, participants identified as CAM-positive underwent further delirium evaluation within the same day by a psychiatrist or psychologist, following the criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders-5th edition (DSM- 5th) (American psychiatric association, 2013). Delirium diagnosis was confirmed when the following DSM-5 criteria were all met: (1) attention disturbance, (2) acute onset with fluctuating course, (3) additional cognitive impairment, (4) the condition cannot be more aptly attributed to another neurocognitive disorder or reduced consciousness and (5) evidence suggesting the disturbance is a direct physiological consequence of another medical condition.
The assessments of POD were rigorously conducted by two trained, departmental professionals, blind to the group allocation of the participants.
Secondary endpoints
Hematological sampling and analytical procedures: Venous blood samples (5 ml) were procured from each participant's arm not designated for infusion at 9:00 AM on T0, T1 and T3. These samples were immediately centrifuged at 4000 revolutions per minute for a duration of 10 minutes. Serum was then meticulously extracted and preserved at -80°C pending analytical assessment. To ensure uniformity across samples, they were normalized to room temperature and homogenized gently before analysis. To curtail variability between tests, all samples underwent concurrent processing within a single day.
Enzyme-Linked Immunosorbent Assay (ELISA) based cytokine analysis
Plasma concentrations of IL-1β, IL-6 and TNF-α were quantified employing Enzyme-Linked Immunosorbent Assay (ELISA) kits supplied by Shanghai Sai Sai Jie Company. Utilizing an advanced double-antibody sandwich Avidin Biotin Peroxidase Complex-ELISA (ABC-ELISA) methodology, the process involved primary monoclonal antibodies targeted against human TNF-α, IL-1β or IL-6. Secondary antibodies incorporated biotin, which subsequently bonded with enzyme-labeled avidin. The introduction of ortho-phenylenediamine induced a color change to yellow, further deepening upon sulfuric acid application. Optical Densities (OD) were measured at a wavelength of 492 nm. The cytokine concentrations correlated directly with the OD readings and were deduced by aligning with established standard curves.
Assay specificity and sensitivity
In this analysis, the employed antibodies demonstrated negligible cross-reactivity with other cytokines, thereby ensuring robust specificity. The assay's sensitivity was defined by the minimum detectable limits, which were established at 1.5 pg/ml for IL-1β, 5 pg/ml for IL-6 and 1.7 pg/ml for TNF-α respectively.
Pain evaluation using the Numerical Rating Scale (NRS)
Postoperative pain intensity was methodically evaluated at T1, T2 and T3 utilizing the Numerical Rating Scale (NRS). Within this scale, "0" denotes the absence of pain, whereas "10" signifies the most intense pain conceivable. For participants experiencing postoperative pain levels of 4 or above on the NRS, pain management was facilitated with an intravenous injection of 40 mg parecoxib sodium.
Adverse events during surgery
Throughout the surgical intervention, continuous monitoring of invasive arterial blood pressure (Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP)), Heart Rate (HR), Electrocardiogram (ECG) and pulse Oxygen Saturation (SpO2) was conducted. The occurrence of intraoperative adverse events, namely hypertension, hypotension, bradycardia and tachycardia was diligently documented. Tachycardia was classified as an HR exceeding 100 bpm, whereas bradycardia was defined by an HR lower than 60 bpm. Hypertension was diagnosed when SBP surpassed 160 mmHg or exhibited an increase exceeding 20% from the baseline. Hypotension was noted when SBP decreased to levels below 90 mmHg or fell by more than 20% from baseline values.
In the event of bradycardia, patients were administered 0.1-0.3 mg of atropine intravenously. Hypotension was managed with either a 4 μg norepinephrine intravenous bolus or a continuous infusion at a rate of 200 μg/h.
Statistical analysis
Sample size calculation and statistical methods: The sample size calculation was based on the incidence of Postoperative Delirium (POD) in a similar patient cohort, reported as 28% in a previous study. We hypothesized a one-third reduction in the incidence of delirium in the DEX group for this trial. With a set significance level of 0.05 and a power of 80%, the required sample size to detect a significant difference was calculated to be 196 using the Pass 11.0 software (NCSS, LLC, Kaysville, Utah, USA). Anticipating an approximate dropout rate of 6%, we set a recruitment target of 208 patients.
Data presentation and statistical analysis
For variables exhibiting normal distribution, we reported means and Standard Deviations (SD). These variables were subjected to independent samples t-tests for comparative analysis. Nonnormally distributed variables were described using median values and interquartile ranges, with the mann-whitney U test employed for statistical assessment. Categorical data were presented as frequencies and percentages, analyzed using either chi-square or fisher’s exact tests, depending on their distribution characteristics.
Levels of pro-inflammatory biomarkers were evaluated through repeated measures Analysis of Variance (ANOVA). All statistical analyses were conducted utilizing Statistical Package for Social Sciences (SPSS) version 22.0 for windows (IBM Corp., Armonk, NY, USA). Two-tailed tests were applied for determining statistical significance, with a Probability (P) value threshold of less than 0.05 deemed indicative of significance.
Data integrity and quality control
To safeguard the integrity and accuracy of data accrued in this study, rigorous monitoring was conducted by the quality control unit of the Clinical Research Ethics Committee at Anting Hospital, Jiading District, Shanghai. This stringent supervision was directed to ensure adherence to the paramount standards of clinical research ethics and methodology, in line with established protocols.
Participants enrollment
This manuscript delineates the results from the Intention-To- Treat (ITT) analysis, with the comprehensive Per-Protocol (PP) analysis detailed in the supplementary materials. Initially, the eligibility of 402 participants was assessed. Out of these, 162 were excluded due to not meeting inclusion criteria or declining to participate. Thus, 240 participants were successfully randomized into two groups, the dexmedetomidine group and the normal saline group, with each group consisting of 120 participants showed in Figure 1.
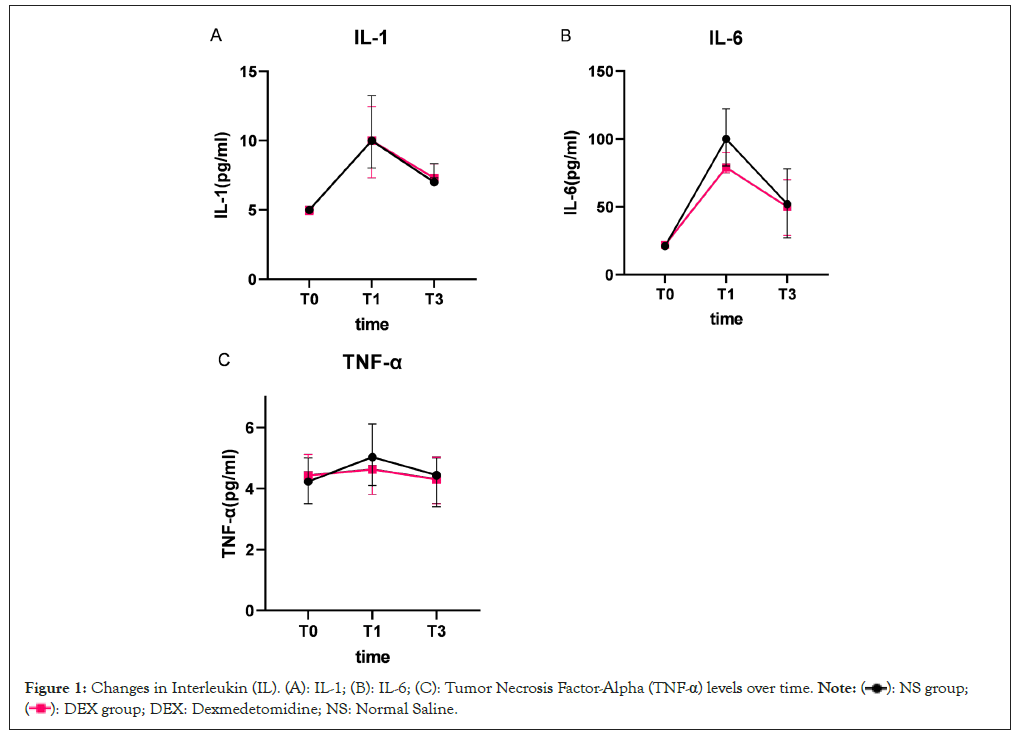
Figure 1: Changes in Interleukin (IL). (A): IL-1; (B): IL-6; (C): Tumor Necrosis Factor-Alpha (TNF-α) levels over time. 
Demographic and clinical history characteristics
In the dexmedetomidine group, participants had a mean age of 78.1 years (SD=6.4), with males comprising 30.8%. The Normal Saline (NS) group exhibited a slightly higher mean age of 79.0 years (SD=6.8), with 31.7% being male. Comparative analyses between the groups indicated no statistically significant differences in key demographic and clinical parameters, encompassing age, sex, Body Mass Index (BMI), educational attainment, American Society of Anesthesiologists (ASA) classification, comorbidities, type of fracture, anesthesia modality, surgical approach, duration of the surgical procedure and the interval between fracture incidence and surgery (all P-values>0.05). It is notable that the use of parecoxib was not required in any participant across both cohorts (Table 1).
| Characteristics | NS group (N=120) | DEX group (N=120) | P |
|---|---|---|---|
| Age (years) | 80.2 ± 7.1 | 79.3 ± 6.9 | 0.652 |
| Gender (female) | 40 | 39 | 0.871 |
| Gender (female) | 80 | 81 | 0.882 |
| BMI (kg/m²) | 24.5 ± 2.8 | 24.0 ± 2.6 | 0.263 |
| Total education years | - | - | 0.322 |
| <6 | 78 (65.0%) | 72 (60.0%) | - |
| 06-Oct | 28 (23.3%) | 31 (25.8%) | - |
| >10 | 14 (11.7%) | 17 (14.2%) | - |
| ASA grade (n%) | - | - | 0.854 |
| I | 10 (8.3%) | 8 (6.7%) | - |
| II | 75 (62.5%) | 76 (63.3%) | - |
| III | 35 (29.2%) | 36 (30.0%) | - |
| Operation time (min) | 62.0 (42.0-72.0) | 61.0 (41.0-71.7) | 0.753 |
| Comorbidities | - | - | - |
| Hypertension | 71 (59.2%) | 70 (58.3%) | 0.912 |
| Diabetes | 26 (21.7%) | 33 (27.5%) | 0.122 |
| Heart failure | 10 (8.3%) | 10 (8.3%) | 1 |
| Lung infection | 2 (1.7%) | 4 (3.3%) | 0.245 |
| Chronic bronchitis | 7 (5.8%) | 8 (6.7%) | 0.802 |
| Atrial fibrillation | 4 (3.3%) | 6 (5.0%) | 0.213 |
| Arrhythmia | 6 (5.0%) | 9 (7.5%) | 0.187 |
| Anesthesia | - | - | 0.238 |
| Subarachnoid block | 96 (80.0%) | 101 (84.2%) | - |
| Combined spinal-epidural | 18 (15.0%) | 17 (14.2%) | - |
| General anesthesia | 6 (5.0%) | 2 (1.7%) | - |
| Type of fracture | - | - | 0.401 |
| Burst fractures | 60 (50.0%) | 50 (41.7%) | - |
| Compression fractures | 58 (48.3%) | 67 (55.8%) | - |
| Flexion-distraction fractures | 2 (1.7%) | 3 (2.5%) | - |
| Surgical procedure | - | - | 0.479 |
| Vertebroplasty | 62 (51.7%) | 55 (45.8%) | - |
| Kyphoplasty | 30 (25.0%) | 29 (24.2%) | - |
| Spinal Fusion | 16 (13.3%) | 21 (17.5%) | - |
| Laminectomy | 12 (10.0%) | 15 (12.5%) | - |
| Time interval between fracture and the operation (days) | 2.8 ± 1.3 | 2.6 ± 1.5 | 0.197 |
Note: NS: Normal Saline; ASA: American Society of Anesthesiologists; BMI: Body Mass Index; DEX: Dexmedetomidine; P: Probability.
Table 1: Demographic and medical history in the Intent-To-Treat (ITT) analysis.
Postoperative delirium incidence and numeric rating scale scores
On the first postoperative day (T1), a marked reduction in the incidence of postoperative delirium was observed in the dexmedetomidine group compared to the Normal Saline (NS) group (13.3% vs. 25.8%, P=0.015). The overall incidence of POD was lower in the dexmedetomidine group (16.7% vs. 30.0%, P=0.015). The subsequent days showed a decrease in POD incidence for both groups, with no significant differences noted on the second (T2) and third (T3) days (3.3% vs. 5.0%, P=0.518; 1.7% vs. 2.5%, P>0.99 respectively). Pain assessments, conducted using the Numeric Rating Scale (NRS), indicated comparable scores between the groups at T1, T2 and T3 (all P-values>0.05) (Table 2).
| NS group (N=120) | DEX group (N=120) | P | |
|---|---|---|---|
| POD | - | - | - |
| T1 | 33 (27.5%) | 18 (15.0%) | 0.025 |
| T2 | 7 (5.8%) | 5 (4.2%) | 0.531 |
| T3 | 4 (3.3%) | 3 (2.5%) | 0.892 |
| Total | 38 (31.7%) | 22 (18.3%) | - |
| NRS | - | - | - |
| T1 | 2.1 (2.0-3.1) | 2.0 (1.9-3.0) | 0.251 |
| T2 | 1.1 (1.0-1.3) | 1.0 (0.9-1.1) | 0.378 |
| T3 | 1.0 (0.9-1.1) | 1.0 (0.9-1.0) | 0.432 |
Note: ITT: Intent-To-Treat; POD: Postoperative Delirium; NS: Normal Saline; DEX: Dexmedetomidine; NRS: Numeric Rating Scale; P: Probability; T1: 1 day after surgery; T2: 2 days after surgery; T3: 3 days after surgery.
Table 2: Incidence of POD and NRS scores after surgery in the ITT analysis.
Inflammatory marker dynamics
At the initial assessment (T0), comparative analysis revealed no significant disparities in the levels of the three examined inflammatory markers between the study cohorts (all P-values>0.05) showed in Figure 1 and Table 3. A congruent trajectory was noted in the serum concentrations of IL-1β across both groups, with a substantial elevation above baseline at both T1 and T3, signifying a pronounced time-dependent augmentation (P<0.001). Relative to baseline (T0), serum IL-6 levels exhibited an upward trend at both T1 and T3. A noteworthy observation was the lower IL-6 concentration in the dexmedetomidine group at T1 compared to the normal saline group, although these differences diminished by T3 (P<0.001). For TNF-α, both groups experienced an increase at T1 and T3. The dexmedetomidine group, however demonstrated significantly reduced TNF-α levels at these time points when juxtaposed with the NS group (P=0.003) (Table 3).
| NS group (N=120) | DEX group (N=120) | F (group)/P-value | F (time)/P-value | F (group*time)/P-value | |
|---|---|---|---|---|---|
| IL-1β | - | - | - | - | - |
| T0 | 5.25 ± 0.66 | 5.16 ± 0.70 | F=0.095 | F=1285.216 | F=0.682 |
| T1 | 11.02 ± 1.50a | 11.20 ± 1.82a | P=0.765 | P<0.001 | P=0.501 |
| T3 | 8.05 ± 1.22ab | 8.04 ± 1.28ab | - | - | - |
| IL-6 | - | - | - | - | - |
| T0 | 23.10 ± 5.20 | 23.85 ± 5.65 | F=235.480 | F=2135.907 | F=32.110 |
| T1 | 106.20 ± 15.50a | 90.10 ± 10.50#a | P<0.001 | P<0.001 | P<0.001 |
| T3 | 64.30 ± 21.00ab | 62.40 ± 16.00ab | - | - | - |
| TNF-α | - | - | - | - | - |
| T0 | 4.40 ± 0.71 | 4.45 ± 0.76 | F=10.780 | F=90.100 | F=6.500 |
| T1 | 5.40 ± 0.83a | 5.00 ± 0.90#a | P=0.001 | P<0.001 | P=0.003 |
| T3 | 4.50 ± 0.88ab | 4.25 ± 0.90#ab | - | - | - |
Note: ITT: Intent-To-Treat; NS: Normal Saline; DEX: Dexmedetomidine; T0: 1 day before surgery; T1: 1 day after surgery; T3: 3 days after surgery; IL: Interleukin; TNF: Tumor Necrosis Factor; P: Probability. (#): P<0.05 vs. the NS group; (a): P<0.05 vs. T0; (b): P<0.05 vs. T1.
Table 3: Levels of IL-1β, IL-6 and TNF-α in the ITT analysis.
Adverse event profile
During the study period, a cumulative count of 102 adverse events was documented, distributed as 44 in the Normal Saline (NS) group and 58 in the dexmedetomidine group. These incidents encompassed conditions such as tachycardia, bradycardia, hypertension and hypotension. Comparative statistical evaluation did not demonstrate significant variance in the frequency of these events between the groups (all P-values>0.05) (Table 4).
| NS group (N=120) | DEX group (N=120) | P | |
|---|---|---|---|
| Tachycardia | 11 (9.2%) | 8 (6.7%) | 0.783 |
| Bradycardia | 17 (14.2%) | 21 (17.5%) | 0.71 |
| Hypertension | 15 (12.5%) | 23 (19.2%) | 0.255 |
| Hypotension | 9 (7.5%) | 11 (9.2%) | 0.611 |
Note: ITT: Intent-To-Treat; NS: Normal Saline; DEX: Dexmedetomidine; P: Probability.
Table 4: Intraoperative adverse events in the ITT analysis.
Study aims and primary outcomes
The objective of this trial was to investigate the impact of dexmedetomidine on postoperative delirium and inflammatory biomarker levels in elderly patients undergoing thoracolumbar compression fracture surgery. The study involved 218 patients, with 110 in the dexmedetomidine group and 108 in the Normal Saline (NS) group. The dexmedetomidine group exhibited a notably lower incidence of POD (18.2%) compared to the NS group (30.6%). There was an initial increase in serum levels of IL-1β, IL-6 and TNF-α at T1, which decreased by T3. Particularly, the levels of IL-6 and TNF-α were lower in the dexmedetomidine group, while IL-1β levels remained consistent across both groups. The frequency of adverse events was comparable between the groups. These results underscore the efficacy of dexmedetomidine in reducing the occurrence of POD on the first postoperative day and in attenuating the early rise in IL-6 and TNF-α levels [21]. Consistent with prior research, the study highlights the link between postoperative delirium and neuroinflammation, stressing the importance of medications impact on inflammatory responses in delirium scenarios [22]. Furthermore, consensus statements on postoperative delirium prevention underscore the importance of exploring pharmacological interventions in mitigating delirium, including the potential effects on pro-inflammatory markers [1]. Therefore, a comprehensive investigation into the effects of dexmedetomidine on postoperative delirium and proinflammatory marker levels is warranted to enhance perioperative management and patient outcomes.
Relevance to existing dexmedetomidine research
Existing literature indicates that the administration of dexmedetomidine reduces the incidence of postoperative delirium in elderly surgical patients. In this study, we monitored serum proinflammatory cytokines IL-1β, IL-6 and TNF-α at various time points to investigate their relationship with dexmedetomidine use and the onset of POD. The relationship between the expression of inflammatory cytokines IL-1β, IL-6 and TNF-α and the use of dexmedetomidine in the development of postoperative delirium is a complex and critical area of investigation. Studies have shown that the activation of the Nuclear Factor-Kappa B (NF-κ B) signaling pathway due to various pathogenic factors can lead to increased expressions of pro-inflammatory cytokines, including IL-6, IL-1β and TNF-α, which are associated with postoperative delirium [23]. Additionally, evidence suggests that preoperative inflammatory markers, including IL-6, IL-8, IL-10 and IL-1β as well as TNF-α, are correlated with the occurrence of POD in patients undergoing surgical procedure [24]. Furthermore, genome-wide DNA methylation profiles have been studied among neurosurgery patients with and without postoperative delirium, demonstrating heightened inflammatory cytokines such as IL‐1β, IL‐6 and TNF-α in the brain following surgical intervention [25].
These findings collectively emphasize the intricate relationship between inflammatory cytokines and the development of postoperative delirium, shedding light on the potential role of dexmedetomidine in modulating the inflammatory response and its impact on POD. However, further research is warranted to elucidate the specific mechanisms through which dexmedetomidine may influence the expression of inflammatory cytokines and subsequently affect the occurrence of postoperative delirium in surgical patients.
It is important to note that while the references provide valuable insights into the relationship between inflammatory cytokines and postoperative delirium, the direct impact of dexmedetomidine on the expression of these cytokines and its association with POD requires dedicated investigation to inform clinical practice and perioperative management. Another study found that postoperative TNF-α, NSE and IL-6 levels were significantly lower in the dexmedetomidine group among patients undergoing robotic-assisted laparoscopic radical cystectomy [26]. Additionally, the combined use of dexmedetomidine and sufentanil postgeneral anesthesia was shown to decrease postoperative IL-6 and TNF-α levels in younger patient [27].
Implications
The findings of this investigation align with the broader body of research suggesting a notable link between systemic inflammatory responses and the development of Postoperative Delirium (POD) in surgical participants, particularly the elderly. This study contributes to this understanding by illustrating that elevated serum levels of key cytokines-IL-1β, IL-6 and TNF-α on the first day following surgery correlate with an increased incidence of POD. Such an observation is critical, as it provides insight into the inflammatory mechanisms potentially driving the onset of delirium in a postoperative setting.
Moreover, by the third postoperative day, a significant trend was observed: as cytokine levels began to normalize, there was a concurrent notable decrease in the rate of POD. This temporal relationship between the decline in pro-inflammatory markers and the reduced incidence of POD not only underscores the potential causative role of these biomarkers in POD pathogenesis but also opens avenues for targeted therapeutic strategies.
Importantly, the efficacy of dexmedetomidine in managing such postoperative complications was highlighted in this study. Dexmedetomidine's role in modulating inflammatory responses and mitigating the occurrence of delirium post-surgery offers a valuable therapeutic option, particularly for elderly participants vulnerable to such postoperative complications. The integration of these findings with current clinical practices could potentially transform the management strategies for POD, emphasizing the importance of monitoring and controlling systemic inflammation as a means to prevent or reduce the severity of delirium in the postoperative phase.
While our study results are promising, further research is warranted to establish a direct link between postoperative delirium and cytokine levels. The pathophysiology of POD is multifaceted, with inflammation and neuro-inflammation playing pivotal roles. Dexmedetomidine, as an α-receptor agonist, has shown potential in POD prevention. Particularly in elderly patients undergoing hip fracture surgery, agitation is common due to trauma and surgical positioning and dexmedetomidine's sedative effects may mitigate this agitation. Based on the provided references, it is evident that dexmedetomidine has been extensively studied in the context of preventing postoperative delirium. Dexmedetomidine, a benzodiazepine, is widely prescribed for managing alcohol withdrawal syndrome and has been associated with a history of usage in patients who developed POD [19,28]. However, it is important to note that the use of dexmedetomidine has also been associated with higher pain intensity during induction of general anesthesia [29].
In contrast, other pharmacological strategies such as dexmedetomidine, melatonin and certain combinations of medications have also shown potential in preventing POD [30-32]. For instance, dexmedetomidine has been found to be effective in preventing delirium in the elderly undergoing major non-cardiac surgery [33]. Similarly, melatonin and its analogues have demonstrated effectiveness in reducing the incidence of POD.
Moreover, it is important to consider the potential risks associated with dexmedetomidine usage. For instance, a study by suggests that the postoperative use of long-acting benzodiazepines, including dexmedetomidine, is associated with a higher risk of postoperative delirium compared to short-acting agents [34]. Additionally, the study by indicates that interference between ketamine and dexmedetomidine at the level of cytochrome P450 enzymes in the liver may occur, highlighting potential drug interactions [35].
The study findings indicate that dexmedetomidine effectively reduces the occurrence of Postoperative Delirium (POD) on the first day following thoracolumbar compression fracture surgery in elderly patients. Furthermore, dexmedetomidine significantly mitigates the short-term elevation of IL-6 and TNF-α levels. These results suggest that dexmedetomidine not only contributes to a lower early POD incidence but also improves short-term recovery in elderly thoracolumbar compression fracture patients. This underscores the potential of dexmedetomidine as a valuable adjunctive tool in perioperative management for this vulnerable patient population, providing dual benefits both psychologically and physiologically. However, caution is advised in the use of dexmedetomidine, given the potential for side effects such as hypotension, bradycardia and cardiac conduction inhibition, especially in this vulnerable population.
Nevertheless, our study has limitations. Being a single-center study, there may exist inherent selection and treatment biases. Consistency in patient management by the same surgical team, while ensuring uniformity, may restrict the generalizability of our findings. Additionally, the study was conducted at a secondary medical center, which may limit the applicability of our results to a broader population of elderly fracture patients. Another limitation is the measurement of pro-inflammatory markers at only three time points, without immediate pre-anesthesia and post-anesthesia sampling. The short-term follow-up period of three days, attributed to brief hospital stays and challenges in postdischarge sample collection, further constrains our conclusions. Although peripheral pro-inflammatory marker levels may reflect central nervous system inflammation, future research should focus on inflammation markers directly related to the brain. Lastly, for patients lost to follow-up with missing NRS scores and blood markers, mean/median imputation methods were employed, potentially impacting study outcomes.
Ethical approval and consent to participate
This research was ethically approved by the Institutional Review Board of Beijing Rehabilitation Hospital, Capital Medical University. All participants provided informed consent, in accordance with the Declaration of Helsinki, ensuring voluntary participation and understanding of the study's objectives and methods.
Consent for publication
Consent for publication was obtained from all participants involved in the study. They were informed that the data collected, while maintaining confidentiality and anonymity, could be used in publications and presentations related to this research.
Availability of data
All materials, data and protocols are present in the manuscript or are available upon request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Authors' contributions and materials
Ye Caimin, serving as the first author, spearheaded the study's conceptualization, methodology design and the drafting of the manuscript. Shen Jian, in the capacity of co-first author, was integral in data acquisition and analysis, contributing substantially to the empirical foundation of the research. Zhang Chengcheng, as the secondary author, played a pivotal role in data interpretation and literature consolidation. Professor Hu Cuiyun, the corresponding author, provided overarching supervision, critical review and final approval of the manuscript, ensuring the scholarly rigor and fidelity of the study. All authors have given their consent for the manuscript's publication.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We would like to thank Peihang XU from the University of Hong Kong for assisting with the preparation and English revision of this manuscript.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Ye C, Shen J, Zhang C, Xu Z, Hu C (2024) Dexmedetomidine's Role in Modulating Postoperative Delirium Incidence and Pro-Inflammatory Cytokine Levels Interleukin-1 Beta (IL-1β), Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α) in Geriatric Thoracolumbar Compression Fracture Surgery Patients. J Anesth Clin Res. 15:1131.
Received: 23-Jan-2024, Manuscript No. JACR-24-29311; Editor assigned: 25-Jan-2024, Pre QC No. JACR-24-29311 (PQ); Reviewed: 09-Feb-2024, QC No. JACR-24-29311; Revised: 16-Feb-2024, Manuscript No. JACR-24-29311 (R); Published: 23-Feb-2024 , DOI: 10.35248/2155-6148.24.15.1131
Copyright: © 2024 Ye C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.