Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2014) Volume 3, Issue 4
By considering the prevalence of depression in subjects with sleep disorders, our purpose is diagnosis of depression in these subjects. Shortened REM latency (i.e, the interval between sleep onset and the occurrence of the first REM period), increased REM sleep duration and increased REM density (i.e., the frequency of rapid eye movements per REM period) have been considered as biological markers of depression, so to diagnose of depression in subjects with sleep disorders we used analysis of EEG signal in REM sleep. The features of frequency and time domains in four channels (C3-C4-O1-O2) and four bands (delta, alpha, beta ,gamma) of REM sleep EEG were compared in 10 depressed (37.5 ± 8.34 years; mean ± standard deviation) and 14 non-depressed (36.6 ± 11.1 years; mean ± standard deviation) subjects with sleep disorders. According to results, most of the significant differences (P<0.05) have related to C3 and O1, in first stage and first period of REM sleep. Our results also show significant increase of beta activity in depressed subjects. Mahalanobis distance has classified 91.7% of the depressed and non-depressed subjects correctly.
Keywords: Depression; Sleep disorder; REM sleep; Sleep EEG signal; Time and frequency analysis
Depression is one of the most commonly occurring of the major psychiatric disorders that profoundly affects individuals' thoughts, emotions, sense of self, behaviors, interpersonal relations, physical functioning, biological processes, work productivity, and overall life satisfaction [1]. The prevalence of this disease in the world today is such that the World Health Organization has predicted that by 2020 depression will be the second leading cause of disease worldwide [2,3]. Depression has symptoms like depressed mood, loss of interest and fatigue, tiredness, cognitive dysfunction, i.e., negative view of the self, negative ruminations, loss of appetite and libido, suicidality and sleep problems. Disturbed sleep is reported by up to 90% of depressed subjects and insomnia or hypersomnia are among diagnostic criteria for depression [4]. Depressed patients complain of insomnia (i.e., prolonged sleep latency, frequent awakenings during sleep, early morning awakening), disturbing dreams and nightmares and bad quality of sleep. These symptoms can accompanied with difficulty in maintaining sleep, decrease of total sleep time and quality, abnormalities of REM sleep, changes in the structure of NREM sleep and variables derived from quantitative sleep electroencephalogram (EEG) analysis [5-8].
Many studies have done on the analysis of sleep EEG signals in depressed patient and demonstrated that REM latency (interval between sleep onset and the occurrence of the first REM sleep period) was shorter in patients with depression and correlated inversely with severity of disease [9,10]. However, this is violated in other cases [7,11] or elsewhere stated that REM latency is associated just with primary depression [12]. Also, there is a phasic change in the activity of REM sleep, especially in the first half of the sleep that the severity of this change could be indicative of depression [13]. Gillin et al. using discriminant analysis and by characteristics such as total sleep time, sleep quality, sleep latency, time of early morning awakening and REM sleep, correctly classified 82% of depressed and normal subject [14]. Borbély et al. have reported that a power spectral density of sleep EEG in 0.25 to 2.5 Hz in depressed patients is lower than normal. They also shown that the power density in the entire frequency range (0.25 to 25 Hz) exhibited a decreasing trend over the first three NREM/REM sleep cycles for both groups, but in each cycle depressed people had lower values than controls [6], although this result was breached in later years by Mendelson et al. [7]. Tekell and colleagues in their work demonstrated increase of high frequencies activity in these patients [15]. It was shown that high frequencies and beta are greater in depressed women compared with depressed men, especially in right hemisphere [16]. Some studies shown that alpha activity decreases in depressed subject [17], although some others have reported increase of alpha and beta activity in depressed patients [18]. Liscome et al. by considering gender shown that generally women in both depressed and normal groups had higher delta amplitude than their male counterparts, and this effect was more common in the first REM period. They also shown that depressed men had lower alpha amplitude than depressed women in all three REM periods and also confirmed higher theta amplitude in the depressed women compared with all other groups for each REM period [19]. Armitage and colleagues suggested that depressed girls had lower delta amplitude than healthy girls in the first NREM sleep [20]. Armitage and colleagues in 1995 and 2001 reported that inter-hemispheric coherence decreases in depressed people [17,18]. Knott and colleagues in 2001 using beta and delta inter-hemispheric coherence, beta intra-hemispheric coherence and alpha intra-hemispheric power asymmetry could classify 91.3% of depressed and normal subjects [21].
Palaginii and colleagues [22] have examined the importance of REM sleep in depression and reported that there is a high correlation between the parameters of REM sleep and depression. Yang Li et al. [23] have shown that depressed patients had more regular brain waves during mental arithmetic than rest and so depressed and normal subject are more separable in the rest situation of brain. They could classify 80% of depressed and normal subjects using discriminant analysis. Because of prevalence of depression in the subjects with sleep disorders, the aim of this study has been diagnosed of depression in these subjects, using analysis of REM sleep Electroencephalogram signals.
Subjects
Twenty four subjects with sleep disorders participated in this study and consent was taken from all of them. By diagnose of psychologist and according to Beck depression inventory (BDI) that was taken from subjects before EEG investigation, 10 persons (37.5 ± 8.34 years; mean ± standard deviation) were depressed with BDI score more than 18 and 14 persons (36.6 ± 11.1 years) were non-depressed with BDI score below 10. These persons only complained of insomnia, hypersomnia, dream and nightmare, excessive daytime sleepiness or poor quality of sleep and none of them had rest leg syndrome (RLS), breathing disorders like apnea or chronic snoring and other sleep disorders. All participants spent one night in the Sleep Disorder Unit of Baharluo Hospital and all of them were medication-free at the time of sleep study for at least 2 weeks.
Polysomnography
Polysomnography includes Electroencephalogram (EEG), Electrooculogram (EOG), Electromyogram (EMG), Electrocardiogram (ECG) and recording of legs movement and breathing signals during sleep. The EEG data that used in this study were collected from C3, C4, O1, and O2 channels referenced to left and right mastoid (A1, A2). Signals were recorded at the sampling frequency of 128 Hz.
Preprocessing
The EEG signals were filtered with 0.5-44 Hz IIR band pass Butterworth filter. Notch filter is also used to remove the 50 Hz frequency. To extract delta, alpha, beta and gamma frequency bands, Butterworth filters with band width of (0.5-4), (8-12 Hz), (14-34 Hz), (36-44 Hz) were used respectively. All of these signal preparations were performed in MATLAB 7.
Sleep staging
In this study, sleep staging has done using EEG of C3, C4, O1 and O2 channels and EOG, based on the Rechtschaffen and Kales criteria [24] by technician of polysomnography and specialist doctor in 30 second epochs of night sleep EEG that was 8 hours on the average. Then all REM sleep stages (set of 30 second epochs of REM sleep with no space) and REM sleep periods (set of REM sleep stages with at least three minutes duration that followed by = 5 consecutive minutes of NREM or wakefulness) have determined in each participants. For statistical purposes, only the first two REM periods and stages were included for analysis because only some of the participants had three or more complete REM periods or stages for the night. Epochs containing movement, breathing or muscle artifacts and NREM sleep or waking epochs were eliminated from the stages or periods of REM sleep. Finally in each person forty-two 30 second epochs, on the average, were saved for later analysis.
Feature extraction
Because of non-stationary nature of EEG signals and existence of artifacts, signals of each channel and in each frequency band first divided to 2 second sections then using Fast Fourier Transform (FFT), power spectrum of each section calculated. Mean, variance, skewness [25], power spectrum threshold and spectrogram threshold criteria [26] for each section calculated and then mean of all section' feature considered as final features. Mean, variance, power and skewness of signals in time domain also considered as features. All features calculated in each frequency band and in four channels. Then magnitude squared coherence (MSC) of EEG signals from different channels in each frequency band determined according to equation (1-2) [26] to measure the degree of coupling between two signals.
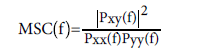 (1-2)
(1-2)
All features have calculated in first and second stages and first and second periods of REM sleep.
ANOVA statistics test and classification
In this paper, after examining data normality by Levene test, features are evaluated using ANOVA test and those with significant difference (P<0.05) were marked. To classifying depressed and non-depressed subjects that all of them had sleep disorders, Mahalanobis classifier with Leave-one-out cross validation, and for applying significant features, ROC curve were used. All parts of this step performed using the version 20 of SPSS software.
Table 1 shows the Results of ANOVA test for the significant features of the first REM sleep stage.
| Features name | Depressed Mean ± Std. Deviation |
Non-depressed Mean ± Std. Deviation |
P value |
| C3_gama_power spectrum skewness | 0.5697 ± 0.0622 | 0.5214 ± 0.0314 | 0.02 |
| C4_delta_ power spectrum skewness | 0.9754 ± 0.0154 | 0.9616 ± 0.0109 | 0.018 |
| O1_beta_mean amplitude of power spectrum | 0.1473 ± 0.0878 (μν2) | 0.0882 ± 0.0458 (μν2) | 0.042 |
| O1_beta_signal variance in time domain | 0.1474 ± 0.0878 (μν2) | 0.0880 ± 0.0456 (μν2) | 0.041 |
| O1_beta_signal power in time domain | 0.0737 ± 0.0439 (μν2) | 0.0440 ± 0.0228 (μν2) | 0.041 |
| O1_beta_spectrogram threshold | 0.1921 ± 0.1237 (μν2) | 0.0821 ± 0.0689 (μν2) | 0.025 |
Table 1: result of ANOVA test for significant features (p<0.05) of first stage of REM sleep
As seen in table, among features of this stage, 6 features had significant difference between groups that most of them are relevant to channel O1. In all these features depressed subjects with sleep disorders had a higher average than non-depressed subjects with sleep disorders.
The result in second stage of REM sleep has shown that only power spectrum skewness in gamma band (mean values and standard deviations of 0.5594 ± 0.0429 and 0.0332 ± 0.5241 respectively for depressed and non-depressed) had significant difference (p<0.05) between groups.
Table 2 shows the results of ANOVA test for the significant features of the first period of REM sleep. In the first period, most significant features were related to the C3 channel, and all the values of the skewness had larger mean in the depressed subjects with sleep disorders compared to non-depressed subjects with sleep disorders.
| Features name | Depressed Mean ± Std. Deviation |
Non-depressed Mean ± Std. Deviation |
P value |
| C3_alpha_ power spectrum threshold | 0.1699 ± 0.0333 (μν2) | 0.2025 ± 0.0406 (μν2) | 0.049 |
| C3_gama_ power spectrum skewness | 0.5674 ± 0.0630 | 0.5240 ± 0.0331 | 0.039 |
| C4_delta_ power spectrum skewness | 0.9748 ± 0.0141 | 0.9620 ± 0.0113 | 0.022 |
| O1- Beta- spectrogram threshold | 0.1842 ± 0.1301 (μν2) | 0.0614 ± 0.0338 (μν2) | 0.016 |
Table 2: Result of ANOVA test for significant features (p<0.05) of first period of REM sleep
By studying the features in second REM sleep period coherence between O1 and O2 in delta band (mean values and standard deviations of 0.83903 ± 0.20813 and 0.50441 ± 0.39057 respectively for depressed and non-depressed) significantly (P<0.05) was more in depressed subjects.
Among the calculated features in the delta and alpha bands, the mean amplitude of the power spectrum at all studied phases of REM sleep and in all four channels C3, C4, O1, and O2 has always been lower in depressed subjects. Checking the power of these bands in time domain also shown the same result, and in most of the cases depressed subjects had lower delta and alpha power but because of greater scattering of these features in non-depressed subjects, no significant difference was shown. The calculation of the mean amplitude of gamma band power spectrum has shown that this feature in the first stage and first period of REM sleep is always more in depressed subjects. The same result was achieved for power of this band in time domain, but because of greater scattering of these features in depressed subjects no significant differences have been shown.
The best classification result using significant features has been obtained 91.7% (sensitivity=90%, specificity=92.9%) , using "power spectrum skewness in gamma band of channel C3 in first stage of REM, power spectrum skewness in delta band of channel C4 in first period of REM, spectrogram thresholds criteria in beta band of channel O1 in first stage and first period of REM and coherence between O1 and O2 in delta band of second period of REM" with area under ROC curve 0.736, 0.764, 0.821, 0.836 and 0.761 respectively.
In this study using processing of REM sleep EEG signal, depression was diagnosed among people with sleep disorders. For this purpose, features of frequency and the time domains were extracted and using Mahalanobis classification, two groups were separated by an appropriate accuracy.
The studying of delta band features has shown that activity of this band in depressed subjects with sleep disorders always was lower, although the difference was not significant. Similar findings have been reported in [19] and in some other researches, decrease of delta activity in first cycle of sleep or in NREM sleep of depressed people also was expressed [6,20]. The results of studying mean amplitude of power spectrum and power of signals in time domain in alpha band revealed that activity of this band has been lower in depressed patients but these differences only approached significance (P=0.05) in first period of REM in channel C3. This is in contradiction with the results of Armitage (2001), which states that alpha activity is greater in depressed people [18]. However their results have related to NREM and total sleep cycle while our results are related to REM sleep and are in accordance with Liscombe's findings [19]. Mean amplitude of power spectrum, power of signals in time domain and threshold of spectrogram of signal in beta band have shown that depressed subjects had higher values and these features had significant differences in first stage of REM in channel O1. The gamma activity in most of the case was higher in depressed subjects but has showed no significant differences in this study. These findings are in agreement with results of [15,19] which reported increase of beta and gamma activity in depressed subjects.
The results showed that skewness of power spectrum in gamma band of channel C3 and in delta band of channel C4 in some stages and periods of REM sleep were significantly higher in depressed group. Studying the coherence between signals in different channels and in different frequency bands reported that inter-hemispheric coherence between C3 and C4 in beta band and total signal although was not significant, but is always lower in depressed subjects and is in agreement with findings of [17,18,21]. Inter-hemispheric coherences between O1 and O2 in all bands were always higher in depressed subjects and had significant difference in delta band of second REM sleep. Using "skewness" and "spectrogram threshold criteria" and "coherence" together, improved the accuracy of classification and at best, mahalanobis classifier with leave-one-out cross validation has separated 91.7% of depressed and non- depressed subjects that in compared with [14,21,23] is a better result. The results of this study indicate that features extracted from REM sleep could classified depressed and non-depressed as well as NREM sleep.
Despite one of the problems of people who referred to sleep disorder clinics is depression, but as far as we know, depression was not diagnosed by analysis of their sleep signals and this research is the first study in this regard. Due to the limitations of the study, the results have obtained by studying the small number of subjects, so the larger population is needed to confirm them. As a future work, it is suggested to study the different age ranges and distinct gender groups with larger populations. Different sleep disorders can also be separately examined.
This research has been supported by Islamic Azad University, Science and Research branch-Tehran-Iran and sleep disorder clinic of Baharlou hospital. We express our appreciation to personnel of sleep disorder clinic of Baharlou hospital for cooperation in collecting data of depressed people with sleep disorders.