Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Introduction: The metabolic syndrome (MS) has been related to the unbalance between pro and anti-inflammatory cytokines; where adiponectin, an anti-inflammatory adipokine, is considered to play a key metabolic role. The consumption of certain micronutrients has been claimed to modify pro and anti-inflammatory cytokines. Aim: To explore whether dietary micronutrients are related with plasma adiponectin in patients with MS. Methods: Cross-sectional analysis. Quartiles of dietary bioactive compounds were compared according to values of plasma adiponectin. Interquartile variation (IQV) and correlation analyses were performed. Results: There was a significant IQV of dietary unsaturated fatty acids (between 50%-66% of change, p25 vs p75, p<0.05), particularly for trans-fatty acids, poly-unsaturated fatty acids, mono-unsaturated fatty acids and ω-6 fatty acids, in relation to plasma adiponectin; as well as a negative correlation (rho=-0.53, -0.37, -0.29 and -0.34, respectively; p<0.05). Conclusion: Dietary amounts of unsaturated fatty acids inversely related to plasma adiponectin in patients with MS.
Adiponectin; Metabolic syndrome; Diet; Fatty acids
The metabolic syndrome (MS) comprises a set of widely distributed risk factors favoring the development of cardiovascular diseases and type 2 Diabetes Mellitus (DM2) [1-4].
Pathophysiological mechanisms underlying MS have been related to the unbalance between pro and anti-inflammatory cytokines; where adiponectin, an anti-inflammatory adipokine, is considered to play a key role due to different effects like: (1) the induction of other anti-inflammatory cytokines like IL-10 and interleukin 1 receptor antagonist (IL-1RA) in human monocytes, monocytederived dendritic cells and macrophages, (2) the regulatory effect on insulin sensitivity, and (3) the anti-atherogenic effect [5-7].
The consumption of pro-inflammatory diets and hyperglycemia have been associated with higher prevalence of hypertension and obesity, suggesting that dietary habits affect metabolic profiles considered of higher risk [6-8].
There is an increasing interest in the study of dietary bioactive compounds with ability to modify plasma cytokines, which has been evidenced throughout several longitudinal studies. For example, the consumption of food from animal source has been observed to decrease plasma adiponectin, whereas the intake of vegetable source foods, enriched with fiber and ω-3 fatty acids, may increase plasma adiponectin [5-7]. Likewise, the consumption of high fiber, complex carbohydrates-enriched diet has been reported to increase plasma adiponectin in overweight and obese subjects, while low fiber, fat-enriched diet reduced plasma adiponectin in patients with DM2 [9,10]. Nevertheless, whether other food properties modify adiponectin is less clear and sometimes controversial. Hypocaloric diets have been reported to increase plasma adiponectin, while other studies have failed to achieve a significant effect of hypocaloric diets on adiponectin [11-14].
Therefore, the present study was designed to explore whether different dietary compounds modify plasma adiponectin in a higher risk population like subjects with MS.
Study design
Observational, analytical cross-sectional study.
Setting
This study was performed at Endocrinology and Bariatric Surgery Departments, as well as Laboratory of Experimental Metabolism and Clinical Research Department, from Centro Médico Nacional “20 de Noviembre”, ISSSTE at Mexico City, between 2017 and 2019.
Study population
Patients older than 18 years old, diagnosed with MS as defined by NCEP/ATP III, meeting at least three of the following components: (1) waist circumference of more than 102 cm in men or more that 88 cm in women, (2) fasting triglyceride level of 150 mg/dL or higher, (3) HDL cholesterol ≤ 40 mg/dL for men or 50 mg/dL for women, (4) systolic/diastolic blood pressure ≥ 130/85 mmHg or receiving drug treatment, and (5) fasting plasma glucose ≥ 100 mg/dL [2]. Patients were excluded if presence of chronic renal failure, hepatic disease, decompensated heart failure, as well as those patients participating in other protocols involving physical rehabilitation or nutritional programs.
For sample size calculation, we estimated the effect of food content on adiponectin plasma concentration, according to previous reports [7], considering 95%CI alpha=0.05, our sample size was n=95, which rendered a beta power of >0.8. The study protocol complied with the Declaration of Helsinki and was approved by the Local Ethics Committee. Informed consent was obtained from all patients for study inclusion.
Anthropometry
Clinical-demographic data were obtained through individual interviews performed by experienced researchers. Anthropometric variables (weight, height, BMI, arm and waist circumferences) were obtained by clinical nutrition specialists, according to standard methods [15].
Dietetic habits
A 24 hour recalls were used to register habitual type of diet, with emphasis in their food schedules and size portions that patients consumed a day before the interview. In case that food consumption on the day before did not reflect the usual diet, patients were asked to describe a habitual day. NASCO® food replicas kit was used to help patients in order to describe the size portions more accurately. All 24-hour recalls were analyzed with the Food Processor® program v.7, allowing estimating the amount of energy (kilocalories), macro and micro-nutrients. All measurements were performed by 2 previously experienced nutritionists.
Adiponectin determination
All blood samples were obtained after 8 hours fasting, by antecubital venopuncture. Then, blood was immediately centrifuged 5000 rpm for 5 min, at room temperature and plasma was recovered. Plasma adiponectin was determined by immunomagnetic multiplexing assay (Milliplex Map Human Adiponectin Magnetic Bead Panel Thermo Fischer Scientific, USA), and readings were performed in a MAGPIX System 40-072-EM (Millipore, Austin, Texas, USA). The measurements were processed according to provider specifications.
Statistical analysis
Normality data analyses were performed with Shapiro-Wilks test. All continuous variables were expressed as mean ± SD or median and InterQuartile Range (IQR, P25, P75), as appropriate; qualitative data were shown as n (%). For inferential analyses, one-tailed, non-paired U-Mann Whitney or T-test were used as appropriate; likewise, Spearman test was used to analyze relation between quantitative data. All statistics tests were performed using software PRISM (v. 6.0) and SPSS (v. 20). Statistical significance was considered if p ≤ 0.05.
The study population was constituted by 95 patients with metabolic syndrome, aged 58 years-old, 78.7% female, with higher prevalence of insulin resistance, dyslipidemia and obesity. Likewise, anthropometric and biochemical parameters indicate cardiometabolic risk, despite general intake of macronutrients was normal (Tables 1-3).
| Age (years-old) | 58.5 ± 9.9 |
| Male (n [%]) | 20.0 (21.3) |
| Co-morbidities | |
| Type 2 Diabetes Mellitus (n [%]) | 76.0 (80.9) |
| Dyslipidemia (n [%]) | 58.0 (61.7) |
| Obesity (n [%]) | 47.0 (50) |
| HAS (n [%]) | 59 (62) |
| Biochemical data | |
| Plasma Glucose (mg/dL) | 106.0 (93.5 to 126.0) |
| Total Cholesterol total (mg/dL) | 142.9 (100.0 to 176.0) |
| c-HDL (mg/dL) | 45.9 ± 14.7 |
| c-LDL (mg/dL) | 92.4 (62.7 to 120.3) |
| Triglycerides (mg/dL) | 134.3 ± 47.1 |
| Adiponectin (pg/dL) | 5.44 (2.28 to 12.68) |
| Note: Quantitative data are resumed as mean ± SD or p50 (p25 to p75) as appropriate; while qualitative data are described as n (%). Abbreviatures: c-HDL: high-density cholesterol; c-LDL, low-density cholesterol. | |
Table 1: Clinical-Demographic Characteristics (n=95).
| Weight (Kg) | 78.3 ± 17.1 |
| BMI (Kg/m2) | 28.9 (26.3 to 32.6) |
| Brachial Circumference (cm) | 32.5 (29.5 to 35.4) |
| Wrist Circumference (cm) | 99.5 (91.2 to 106.0) |
| Hip Circumference (cm) | 102.0 (96.3 to 111.8) |
| Note: Quantitative data are resumed as mean ± SD or p50 (p25 to p75) as appropriate; while qualitative data are described as n (%). Abbreviatures: BMI, Body Mass Index. | |
Table 2: Anthropometric Characteristics.
| Energy (Kcal) | 1430.5 (1083.7 to 1874.1) |
| Proteins (g) | 71.4 ± 27.5 |
| Carbohydrates (g) | 185.0 (116.5 to 252.8) |
| Lipids (g) | 51.2 ± 30.5 |
| Note: Quantitative data are resumed as mean ± SD or p50 (p25 to p75) as appropriate; while qualitative data are described as n (%). Abbreviatures: BMI, Body Mass Index. | |
Table 3: Macronutriments Consumption.
The study population was divided by quartiles according to the amount of nutrient composition in the diet. Then, value of plasma adiponectin was distributed between the quartiles of nutrients.
A significant progressive reduction of adiponectin across the increasing quartiles of nutrients was observed for trans-fatty acids, PUFAs, mono-unsaturated fatty acids and ω-6 fatty acids (Table 4).
| - | Plasma adiponectin (pg/dL) according to micronutrients | % Change P25 vs P75 | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p-value | - | p-value | |
| Trans- FA (g) | 7.2 (4.4 to 10.5) | 4.5 (2.9 to 7.4) | 4.5 (2.3 to 7.8) | 2.4 (1.9 to 4.8) | 0.01 | -66.62 | 0.01 |
| PUFA (g) | 6.2 (2.2 to 10.9) | 7.1 (4.0 to 11.9) | 3.0 (2.3 to 5.8) | 2.6 (2.1 to 4.5) | 0.004 | -57.95 | 0.01 |
| Mono- unsaturated FA (g) | 5.6 (2.2 to 9.9) | 5.6 (3.0 to 9.8) | 3.6 (2.3 to 6.1) | 2.6 (1.6 to 4.9) | 0.06 | -53.75 | 0.03 |
| w-6 FA (g) | 6.7 (2.3 to 9.9) | 6.2 (3.0 to 10.6) | 4.0 (2.2 to 6.2) | 3.3 (2.3 to 4.5) | 0.05 | -50.97 | 0.04 |
| Potassium (mg) | 3.0 (2.1 to 11.9) | 5.4 (3.0 to 9.5) | 4.5 (2.6 to 7.2 | 5.4 (2.2 to 6.7) | 0.49 | 77.74 | 0.88 |
| Calcium (mg) | 6.5 (3.8 to 12.8) | 4.5 (2.4-6.9) | 3.8 (2.2 to 5.7) | 3.3 (2.3 to 7.7) | 0.14 | -49.15 | 0.05 |
| Vitamin B6 (mg) | 5.8 (3.9 to 6.9) | 4.5 (1.9 to 9.0) | 4.4 (2.2 to 8.8) | 3.0 (2.4 to 4.9) | 0.23 | -47.77 | 0.04 |
| Vitamin B2 (mg) | 6.3 (3.9 to 9.4) | 4.5 (2.3 to 8.3) | 4.5 (1.9 to 7.7) | 3.3 (2.4 to 7.0) | 0.37 | -47.61 | 0.07 |
| Cholesterol (mg) | 5.2 (4.0 to 9.9) | 5.4 (2.2 to 7.2) | 4.5 (2.3 to 8.3) | 2.9 (2.3 to 6.5) | 0.45 | -42.33 | 0.08 |
| Vitamin E (UI) | 7.0 (2.5 to 12.0) | 5.4 (2.4 to 10.6) | 4.1 (2.3 to 7.0) | 5 (2.3 to 5.4) | 0.25 | -41.46 | 0.06 |
| Vitamin C (mg) | 4.0 (1.5 to 7.1) | 3.8 (2.6 to 5.4) | 3.8 (2.6 to 5.4) | 5.8 (2.6 to 9.1) | 0.82 | 40.59 | 0.88 |
| Vitamin B1 (mg) | 5.6 (3.7 to 9.9) | 3.8 (2.0 to 6.5) | 4.5 (2.0 to 8.9) | 3.5 (2.2 to 6.5) | 0.37 | -36.96 | 0.04 |
| Sodium (mg) | 6.2 (2.7 to 9.9) | 3.1 (2.1 to 5.4) | 4.3 (2.4 to 8.1) | 4.1 (2.8 to 8.0) | 0.1 | -33.6 | 0.41 |
| Carbohydrates (g) | 5.2 (2.4 to 9.9) | 4.5 (2.3 to 6.6) | 4.1 (2.1 to 7.1) | 3.7 (2.3 to 8.5) | 0.9 | -28.6 | 0.58 |
| Vitamin B3 (mg) | 5.4 (2.3 to 6.8) | 5.0 (3.1 to 1.7) | 2.5 (2.1 to 8.9) | 4 (2.5 to 5.8) | 0.49 | -26.65 | 0.4 |
| w-3 FA (g) | 4.5 (1.9 to 6.8) | 5.4 (2.3 to 9.4) | 5.4 (2.9 to 7.5) | 3.9 (2.5 to 6.1) | 0.57 | -14.61 | 0.84 |
| Selenium (mcg) | 5.0 (2.2 to 10.2) | 5.4 (3.4 to 8.3) | 3.8 (2.2 to 8.9) | 4.5 (2.4 to 5.6) | 0.55 | -10.1 | 0.34 |
| Folate (mcg) | 4.3 (2.3 to 9.1) | 3.9 (2.9 to 6.4) | 5.8 (1.7 to 9.6) | 3.8 (2.3 to 6.2) | 0.57 | -6.86 | 0.57 |
| Satured FA (g) | 4.7 (2.1 to 9.4) | 4.7 (2.1 to 9.4) | 3.1 (2.3 to 6.1) | 4.9 (2.1 to 12.0) | 0.45 | 3.16 | 0.99 |
| Vitamin D (UI) | 3.8 (2.2 to 11.5) | 6.3 (3.9 to 7.5) | 2.3 (1.7 to 4.6) | 5.4 (2.8 to 6.0) | 0.16 | 0 | 0.81 |
| Note: Adiponectin was distributed according to quartiles of dietary micronutrients (Q1 refers to <p25; Q2 refers to p25-50; Q3 refers to p50-p75 and Q4 refers to >p75). Interquartile viariance was compared by Kruskall-Wallis test. Then, the magnitude of change was estimated by U-Mann-Whithney test. Abbreviatures: FA, Fatty Acids; PUFA, Poly-unsaturated fatty acids. | |||||||
Table 4: Dietary Micronutrients and Plasma Adiponectin.
The highest correlations between nutrients amounts and plasma adiponectin are shown in Figure 1, highlighting the moderate negative correlation between trans-fatty acids (-0.53, CI95% -0.70 to -0.31, p=0.0001); PUFA (-0.37, CI95% -0.57 to -0.12, p=0.0019); ω-6 fatty acids (-0.33, CI95% -0.54 to 0.08, p=0.0043) and monounsaturated fatty acids (-0.29, CI95% -0.51 to 0.03, p=0.012) with plasma adiponectin.
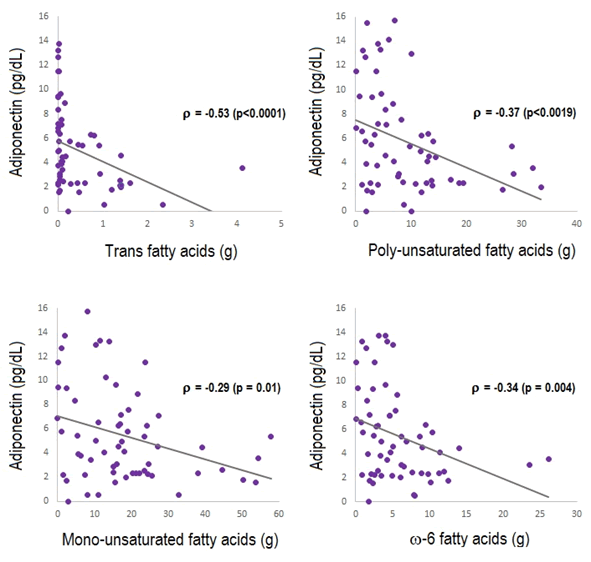
Figure 1: Dietary components most related with plasma adiponectin. The figure shows the correlations between micronutrients most significantly related to plasma adiponectin. Spearman correlation.
Our main finding was the inverse relation adiponectin with diet amount of unsaturated fatty acids, particularly trans-fatty acids, poly-unsaturated fatty acids, mono-unsaturated fatty acids and ω-6 fatty acids.
Our results are similar to observations reported by Esposito et al., where consumption of a high fat, low fiber diet for 1 week induced a reduction of plasma adiponectin, either in healthy individuals and patients with DM2 [10].
In the other hand, Peake et al. showed that consumption of a saturated fat-rich breakfast for approximately 1 week did not significantly modified plasma adiponectin in healthy individuals or patients with DM2; while Paniagua et al. did not observed difference in mRNA adiponectin between consumption of saturated vs unsaturated fatty acid-rich breakfast for 3 days in healthy individuals with metabolic risk factors. Such controversies with our findings may be explained by variations in the study design, methods to measure adiponectin, the type of fatty acids and time of consumption, suggesting that the effect of unsaturated fatty acids on adiponectin is specific and may depend on the time of consumption, for at least 1 week [16,17].
Our findings are in line with some mechanisms proposed to explain why specific unsaturated fatty acids, calcium and piridoxin may decrease adiponectin. PUFA may modulate enzymes responsible for adiponectin expression through AMPK pathway in the liver and adipocytes; or peroxidation particularly of linoleic acid produces hydroxyalkenal 4-hydroxynonenal derivate, which may reduce adiponectin gene [18,19]. Other responsible mechanisms may be related with calcium and piridoxin. There are reports showing that calcium-content reduces adiponectin, which may be explained that calcium reduces production of fatty acid and promotes lipolysis; whereas piridoxin may be modulating intracellular calcium signaling and lipid metabolism regulation [20].
Our findings contribute to the understanding of how diet composition and the time of specific diet consumption are able to induce higher adiponectin and the associated cardiometabolic benefits. However, some limitations of the study should be considered. First, the heterogenicity of the population regarding life styles, co-morbidities and drug therapies; while a healthy control group was not included. Second, the low sample size, which may be a source for potential bias. Third, the nutrients content in the diet was estimated through a 24-hour recall approach, whereas a direct measure of nutrients would be more accurate.
In conclusion, the results obtained from the present study suggest that diet content of non-saturated fatty acids decreases plasma adiponectin in patients with metabolic syndrome.
The authors declare no conflict of interest. This project was supported by E-015 Program ISSSTE.
The study was designed and performed according to ethical guidelines of the 1975 Declaration of Helsinki, and it was approved by the Local Committees of Research, Ethics in Research and Biosafety of the Centro Médico Nacional ‘20 de Noviembre’ ISSSTE, Mexico City. All participants provided written informed consent.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
This project was supported by E-015 Program ISSSTE.
The authors acknowledge the support from E-015 Program ISSSTE.
Citation: Cuenca JAS, DÃaz-Jiménez DE, Pineda-Juárez JA, Mendoza-Mota AG, Valencia-Aldana OD, Núñez-Angeles S, et al. (2021) Diet Content of Non-saturated Fatty Acids Decreases Plasma Adiponectin in Patients with Metabolic Syndrome. J Nutr Food Sci. 11:817.
Received: 09-Aug-2021 Accepted: 23-Aug-2021 Published: 30-Aug-2021 , DOI: 10.35248/2155-9600.21.s6.1000817
Copyright: © 2021 Cuenca JAS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This project was supported by E-015 Program ISSSTE.