Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2021)
Nile tilapia (Oreochromis niloticus L.) is indigenous species to Ethiopia and constitutes major proportion in the country’s fish production. In an attempt to select better performing strains for aquaculture development, tilapia populations from different Ethiopian rift valley lakes showed different growth performances in pond culture. Investigation of desired culture characteristics of target tilapia populations is required to improve their productivity in aquaculture system. The current study was made to investigate phenotypic characters of the tilapia populations in three geographically isolated Ethiopian rift valley lakes (Chamo, Koka and Ziway). A total of 450 adult tilapias of commercial catches were sampled from the three lakes and their phenotypic characters were analyzed during May 2018 to March 2019. Twenty six morphometric character indices, eight meristic counts, total length, standard length, total weight, length-weight relationship and Fulton’s condition factor were considered in the analysis. The results revealed significant differences (p ≤ 0.05) in most of the morphometric character indices, meristic counts, mean length and weight and Fulton’s condition factor among the three tilapia populations. Chamo tilapia population were found to have highest mean values of total weight, total length and standard length while Koka population have highest mean value of Fulton’s condition factor and positive allometric growth as characters desired in aquaculture. Further research is required to investigate whether the fish populations could maintain those characters in pond cultures.
Aquaculture; Geographically isolated; Meristic; Morphmetric
Nile tilapia (Oreochromis niloticus L.) is cultured worldwide and currently ranked second only to carps in global farmed food fish. The fish is indigenous species to Ethiopia and constitutes major proportion (65%) in the country’s fish production [1]. In the aquaculture development efforts of Ethiopia, wild O. nilotcus were collected from natural environments, especially from the Ethiopian rift valley lakes and stocked into ponds. In an attempt to select better performing strains for aquaculture, tilapia populations from different Ethiopian rift valley lakes showed different growth performances in pond culture. Growth performances of four O. niloticus populations of Ethiopian rift valley lakes (Hawassa, Chamo, Koka and Ziway) were evaluated in pond culture at Batu Fishery and other Aquatic Life Research Center [2] where the Chamo population was found to grow significantly faster than others. Similar studies were conducted to evaluate the growth performances of the tilapia populations of Ethiopian rift valley lakes in pond culture at different times for different experimental periods and found variation in growth performances [3,4] whereby the Koka and Ziway tilapia populations were mentioned as promising candidate populations for aquaculture development in Ethiopia compared to tilapia populations of Hora, Hawasa and Metehara lakes in Figure 1-3.
Figure 1: Location of the Ethiopian rift valley lakes.
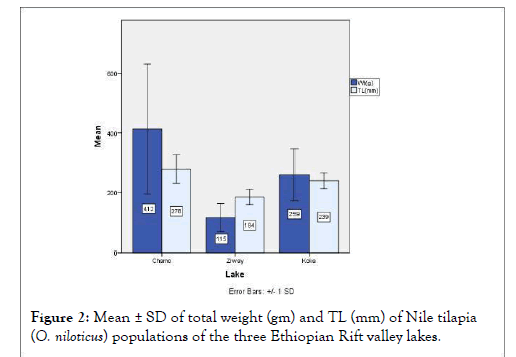
Figure 2: Mean ± SD of total weight (gm) and TL (mm) of Nile tilapia (O. niloticus) populations of the three Ethiopian Rift valley lakes.
Figure 3 : O. niloticus samples of lakes Chamo (left), Koka (middle) and Ziway (right).
The three lakes, Chamo, Koka and Ziway are geographically isolated situated in different sub-drainage basins: Abaya-Chamo, Ziway-Shala and Awash basins, respectively, all within the Ethiopian Rift Valley [5]. Chamo has more diversified ichtyo fauna than other lakes in Ethiopia except Lake Abaya. There are six important fish species for fishery in Chamo. These, according to LFDP [6] are Nile perch (Lates niloticus), Nile tilapia (Oreochromis niloticus), labeo (Labeo horie), African catfish (Clarias gariepinus), Bagrus (Bagrus docmak ) and Barbus (Labeobarbus intermedius). The O. niloticus, L. niloticus and C. gariepinus were numerically the three dominant species in the fish landings [7]. Koka harbors three commercially important fish species: Nile tilapia (Oreochromis niloticus), common carp (Cyprinus carpio) and African catfish (Clarias gariepinus) where O. niloticus were found in healthy state, C. gariepinus have shown signs of growth overfishing and C. carpio have shown under exploitation [8]. Indigenous fish species such as Nile tilapia (Oreochromis niloticus), Barbus (Labeobarbus intermedius), straightfin barb (Barbus paludinosus) and Garra (Garra dembecha), and introduced fish species such as African Catfish (Clarias gariepinus), Crucian carp (Carassius carassius), Common carp (Cyprinus carpio) and Tilapia zillii (Coptodon zillii) are found in Lake Ziway [9]. Among these species, Nile tilapia, African catfish, Crucian carp and Common carp are commercially important ones. Studies indicated [9] that the current annual fish production from Lake Ziway is declining, particularly the population of the Nile tilapia declined tremendously
Aquatic environment of the three lakes in terms of their basic morphometric features, altitude, water quality parameters and fish species diversity are different [10]. Under these different environmental conditions of the three lakes, tilapias are expected to develop different phenotypic and genetic characters. Phenotypic variation is wide in organisms spread in different geographical locations, and often involves ecologically relevant behavioral, physiological, morphological and life historical traits. Phenotypic plasticity can be inclusively defined as the production of multiple phenotypes from a single genotype, depending on environmental conditions [11].
Characterization of the tilapia population helps in utilization of the resource in aquaculture, and further contributes to protect the population in their natural environment. Hence, the objective of the present study was to investigate desired culture characteristics of O.niloticus of rift valley lakes in order to improve their productivityin aquaculture system with specific objective of characterizing theO.niloticus populations of Chamo, Koka and Ziway Lakes based ontheir phenotypic characters.
Description of the study areas
The fish were sampled from three Ethiopian rift valley lakes: Chamo, Koka and Ziway lakes from May, 2018 to March 2019. These water bodies were selected for their targeted Nile tilapia (O.niloticus.) populations to be used in the development of aquaculture in Ethiopia. Lake Chamo is situated in South most part of the Ethiopian rift valley lakes. The lake has surface area of about 297 km2 [12] and recharged mainly by a feeder river called Kulfo. Lake Koka is a manmade reservoir located at about 90 km Southeast of Addis Ababa between 8° 18' 57" to 8° 28' 21" N and 39° 0' 0" E to 39° 9'18" E at an altitude of 1,590 m.a.s.l. It was constructed on Awash River in 1960 for thepurpose of hydro-electric power generation.
The reservoir has a surface area of about 255 km2 with amaximum and mean depth of 14 m and 9 m, respectively [6]. Lake Ziway, spelled also as Zeway, Zway or Zwai (locally called as Lake Danbal) is located in the Ethiopian rift valley between 7° 52’ to 8° 8’N and 38° 40’ to 38° 56’E at an altitude of 1636 m a.s.l. with maximum length of 32 km and maximum width of 20km [6]. The lake is the second largest lake in Ethiopian rift valley after Abaya Lake, having an area of 434 km2 and shoreline length of 137 km. The Lake is, however, the shallowest of the rift valley lakes with maximum and mean depth of 8.95 m and 2.5 m, respectively [6]. The lake has two feeder rivers, Meki River from Northwest and Katar River from East side, and has one outlet river in its Southern part, the Bulbula River, which seasonally flows into Abijata Lake.
Phenotypic characterization of the Oreochromis niloticus
Morphometric characters and meristic counts following described methods [11] were recorded from at least 150 fish samples of each of the tilapia populations of the three study lakes in Table 1.
| A. Morphometri analysis | |||
|---|---|---|---|
| Characters | Acronyms | Characters | Acronyms |
| Total length | TL | Caudal peduncle length | CPL |
| Standard length | SL | Caudal peduncle width | CPW |
| Head length | HL | Caudal peduncle depth | CPD |
| Body depth | BD | Pectoral fin length | Pec FL |
| Body width | BW | Dorsal fin base length | DFBL |
| Head width | HW | Pelvic fin base length | Pel FBL |
| Abdomen length | AL | Length of longest dorsal fin spine | LLoDFS |
| Orbit diameter | OD | Length of last dorsal fin spine | LLaDFS |
| Pre-orbital length | Pr-OL | Length of longest dorsal fin ray | LLoDFR |
| Post-orbital length | Po-OL | Length of last dorsal fin ray | LLaDFR |
| Trunk length | TrL | Length of longest anal finspine | LLoAFS |
| Pelvic fin length | Pel FL | Length of first anal fin spine | LFiAFS |
| Anal fin baselength | AFBL | Length of longest anal fin ray | LLoAFR |
| Caudal fin length | CFL | Length of last anal fin ray | LLaAFR |
| B. Meristic analysis | |||
| Characters | Acronyms | Characters | Acronyms |
| Dorsal fin spines count | DFSC | Pectoralfin rays count | Pec FRC |
| Dorsal finrays count | DFRC | Anal fin spines count | AFSC |
| Pelvic fin spines count | Pel FSC | Anal fin rays count | AFRC |
| Pelvic fin rays count | Pel FRC | Caudal fin rays count | CFRC |
Table 1: Morphometric and meristic characters of O. niloticus populations of the three lakes.
Along with the morphometric measurements, weight (TW) of each specimen was measured to the nearest 0.1 g. At least 150 fishes per lake were assessed. Condition factor of each fish was measured using the following equation:
K=100 (TW/TL3)
Where, k=Fulton’s condition factor
TW=total weight in grams
TL=total length in centimeter
The fish were characterized based on the morphometric and meristic parameters. Values for the quantitative traits of the populations were expressed as mean and standard deviation as mean ± SD. Differences in mean values of the traits between the populations were analyzed in one-way analysis of variance (ANOVA) at significance level of p ≤ 0.05 using Tukey’s mean separation method [13,14].
In this phenotypic characterization, parameters including morphometric character indices, meristic counts, total weight total length, standard length and fish condition factor were analyzed from a total of 450 samples: 150 fish samples for each of the populations in Table 2.
| Characters | Population | Pel FRC | Pel FRC | |||
|---|---|---|---|---|---|---|
| Chamo | Koka | Ziway | ||||
| Morphometric indices | ||||||
| HL/SL | 0.324 | ±0.02 | 0.312 | ± 0.01 | 0.324 | ± 0.02 |
| BD/SL | 0.392 | ±0.02 | 0.360 | ± 0.02 | 0.370 | ± 0.02 |
| BW/SL | 0.158 | ±0.01 | 0.170 | ± 0.01 | 0.167 | ± 0.02 |
| HW/HL | 0.550 | ±0.03 | 0.559 | ± 0.03 | 0.553 | ± 0.10 |
| AL/SL | 0.302 | ±0.02 | 0.314 | ±0.03 | 0.304 | ± 0.02 |
| OD/HL | 0.209 | ±0.03 | 0.220 | ±0.03 | 0.249 | ±0.09 |
| Pr-OL/HL | 0.320 | ±0.03 | 0.287 | ± 0.03 | 0.312 | ± 0.24 |
| Po-OL/HL | 0.497 | ±0.03 | 0.493 | ± 0.03 | 0.484 | ± 0.09 |
| TrL/SL | 0.681 | ± 0.03 | 0.710 | ± 0.02 | 0.690 | ± 0.06 |
| PelFL/SL | 0.257 | ±0.02 | 0.273 | ± 0.05 | 0.263 | ± 0.02 |
| AFBL/SL | 0.194 | ±0.01 | 0.195 | ± 0.01 | 0.182 | ± 0.01 |
| CFL/TL | 0.200 | ±0.01 | 0.201 | ± 0.01 | 0.203 | ± 0.11 |
| CPL/TL | 0.119 | ±0.01 | 0.113 | ± 0.01 | 0.120 | ± 0.01 |
| CPW/TL | 0.040 | ± 0.00 | 0.044 | ± 0.01 | 0.040 | ± 0.01 |
| CPD/TL | 0.105 | ± 0.01 | 0.098 | ± 0.01 | 0.096 | ± 0.01 |
| PecFL/SL | 0.365 | ± 0.02 | 0.342 | ± 0.04 | 0.359 | ± 0.02 |
| DFBL/SL | 0.598 | ± 0.03 | 0.622 | ± 0.06 | 0.601 | ± 0.03 |
| PelFBL/SL | 0.056 | ± 0.01 | 0.052 | ± 0.01 | 0.055 | ± 0.01 |
| LLoDFS/SL | 0.156 | ± 0.01 | 0.141 | ± 0.02 | 0.149 | ± 0.01 |
| LLaDFS/SL | 0.156 | ± 0.01 | 0.141 | ± 0.02 | 0.149 | ± 0.01 |
| LLoDFR/SL | 0.250 | ± 0.03 | 0.224 | ± 0.03 | 0.217 | ± 0.02 |
| LLaDFR/SL | 0.077 | ± 0.01 | 0.082 | ± 0.01 | 0.080 | ± 0.01 |
| LLoAFS/SL | 0.151 | ± 0.01 | 0.138 | ± 0.02 | 0.149 | ± 0.02 |
| LFiAFS/SL | 0.064 | ± 0.01 | 0.050 | ± 0.01 | 0.056 | ± 0.01 |
| LLoAFR/SL | 0.226 | ± 0.02 | 0.221 | ± 0.03 | 0.215 | ± 0.01 |
| LLaAFR/SL | 0.075 | ± 0.01 | 0.081 | ± 0.01 | 0.076 | ± 0.01 |
| Meristic counts | ||||||
| DFSC | 16.59 | ± 0.49 | 16.82 | ± 0.39 | 16.85 | ± 0.37 |
| DFRC | 12.29 | ± 0.49 | 12.21 | ± 0.41 | 12.09 | ± 0.46 |
| Pel FSC | 1.00 | ± 0.00 | 1.00 | ± 0.00 | 1.00 | ± 0.00 |
| Pel FRC | 5.00 | ± 0.00 | 5.00 | ± 0.00 | 5.00 | ± 0.00 |
| Pec FRC | 13.06 | ± 0.35 | 12.63 | ± 0.51 | 12.42 | ± 0.56 |
| AFSC | 3.00 | ± 0.00 | 3.00 | ± 0.00 | 3.00 | ± 0.00 |
| AFRC | 9.29 | ± 0.47 | 9.53 | ± 0.54 | 9.48 | ± 0.51 |
| CFRC | 17.46 | ± 0.88 | 17.23 | ± 0.98 | 17.93 | ± 0.61 |
| TW(g) | 412.24 | ± 218.21 | 259.17 | ± 87.65 | 115.13 | ± 47.14 |
| TL(mm) | 278.15 | ± 47.89 | 239.00 | ±26.35 | 184.43 | ± 24.61 |
| SL(mm) | 225.63 | ± 41.73 | 191.74 | ±22.26 | 149.44 | ± 20.59 |
| Condition factor (K) | 1.78 | ±0.16 | 1.83 | ±0.22 | 1.75 | ± 0.14 |
Table 2: Means and standard deviations of quantitative phenotypic traits based on morphometric character indices and meristic counts.
Nile tilapia population of Lake Chamo were characterized by highest mean weight (412.24 ± 218.21 g), total length (27.81 ± 4.79 cm) and standard length (22.56 ± 4.17 cm), which differed significantly (p ≤ 0.05) from similar parameters of populations of lakes Koka and Ziway. The current mean total length of tilapia population of Lake Chamo is within the range of 19-43 cm length reported [7]. The Chamo population have also scored highest morphometric character indices of BD/SL,CPD/TL,Po-OL/ HL,PelFBL/SL,LLoDFS/SL,LLaDFS/SL,LLaDFR/SL,FiAFS/ SL,LLoAFR/SL and highest record of meristic character in PecFRC in which all the characters differed significantly (p ≤ 0.05) from the mean values measured from the populations of lakes Koka and Ziway. However, the population in Lake Chamo scored significantly (p ≤ 0.05) lowest mean values of BW/SL,TL/ SL and LLaDFR/SL from morphometric indices, and DFSC and AFRC from meristic characters. Generally, O. niloticus population from Lake Chamo were found to be larger in total length and weight, shorter at trunk region with respect to their standard length, dorso-vetrally deep, laterally compressed in body form as compared to other populations. They also had longest pre-orbital region, dorsal and anal fins as indicated in their morphometric indices.
Nile tilapia populations of Lake Koka scored mean weight of 259.17 ± 87.65 g, mean total length of 23.90 ± 2.63 cm and standard length of 19.17 ± 2.22 cm; values which were significantly (p ≤ 0.05) lower than that of Chamo population but higher than that of Ziway population. A mean total length that a fish of a given stock would reach if they were to grow indefinitely (L∞) was estimated to reach 44.5 cm for the O. niloticus population of Koka [8]and 28.1 cm for the O. niloticus population of Ziway [15]. Thesize differences between the population means in the current resultagrees with the above reports. Highest mean score of Fulton’scondition factor (K) was obtained in population of Lake Koka (1.83) and the value differed significantly (p ≤ 0.05) from that of the populations of lakes Chamo (1.78) and Ziway (1.75). The average K-values of O. niloticus were reported to vary over time; 1.87 for Koka population [16] and 1.89 [17] and 1.81 [15] for Ziway and 2.35 for Chamo [18]. The population of Lake Koka scored highest morphometric character indices of AL/SL, TL/ SL, DFBL/SL, CPW/TL, PelFL/SL and LLaAFR/SL but lowest mean values of HL/SL, BD/SL, CPL/TL, PecFL/SL, PelFBL/ SL, LLoDFS/SL, LLaDFS/SL, LLoAFS/SL, and LFiAFS/SL which were significantly different (p ≤ 0.05) from that of lakes Chamo and Ziway populations. As compared to populations of lakes Chamo and Ziway, the Koka O. niloticus population were generally found to have longer trunk region, laterally wide (fat), not dorso-ventrally deep, with shorter head and fin spines.
The tilapia population of Ziway were significantly (p ≤ 0.05) lower in their mean total weight (115.13 ± 47.14 g), mean total length (18.44 ± 2.46 cm) and standard length (14.94 ± 2.06 cm) than the lakes Chamo and Koka populations. The population had also lowest morphometric character indices of LLoDFR/SL, AFBL/SL, LLoAFR/SL and the lowest meristic counts of dorsal and pectoral fin rays which were significantly (p ≤ 0.05) different from mean values observed in that of Lakes Chamo and Koka populations. However, the population scored the highest mean value of OD/HL morphometric character index and the highest mean caudal fin ray count and differed significantly from that of lakes Chamo and Koka. Generally, O. niloticus population of Lake Ziway was relatively smaller in size, have shorter dorsal fin rays and wider orbit diameter with respect to their head length.
Morphometric characters in fish have been demonstrated to be influenced by environmental factors [19]. Chamo and Ziway are natural lakes while Koka is an artificial lake constructed for hydro-electric power generation. Aquatic environment of the three lakes in terms of their basic morphometric features, altitude, water quality parameters and fish species diversity are different [10]. The O.niloticus populations of the three lakes are accordingly isolatedfor centuries and developed different morphometric characters toadapt to their corresponding environmental conditions.
Fulton’s condition factor (K), which indicates the nutritional level and status of the fish over time, might be greatly influenced by environmental factors. The higher Fulton’s condition factor (K), observed in O. niloticus population of Lake Koka in the current study could be resulted as a function of better feed availability in artificial reservoirs than in natural lakes. Though the degree vary from lake to lake, anthropogenic impacts such as pollution due to silt and nutrient load from watershed areas, municipal waste, industries (tannery, flower culture), water level fluctuation and fishing pressures have directly or indirectly affected the fish community in the lakes [20]. Floodplains associated with the reservoirs are generally known to be most productive aquatic ecosystems. The K value of O. niloticus population of lakes Chamo, Koka and Ziway varied over time based on environmental changes [16-18].
Phenotypic adaptations of fish to their environment do not necessarily cause a change in genetic constitutions of the population [21] and hence phenotypic differences among populations cannot usually be taken as evidence of genetic differentiation. However, the O. niloticus populations of the three lakes are isolated for geological time that development of genetic differences among the populations is most likely. In genetic analysis using total mtDNA digestions, Seyoum and Kornfield [22] grouped O. niloticus of Ethiopia into different subspecies: O. n. cancellatus (Awash basin), O. n. filowa (Sodore hot springs), O. n. taita (Tana population) [23]using allozyme, clustered natural populations of O. niloticus inthe Ethiopian Rift valley (Lakes Ziway, Hawasa, Koka and Sodorehot springs) in one group and populations of Tana, Nile, Kenya inother group. The authors [20] considered the subspeciesclassification made by the former study [22] was premature.According to the species clustering of the later authors [23], the O.niloticus of Lake Chamo can be related to the Kenyan and Nilegroup since the Abaya-Chamo basin connects down to the ChewBahir and Turkana Lake in northern Kenya.
In a study of phylogenetic differentiation of wild and cultured Nile tilapia populations based on phenotype and genotype analysis [11], it was reported that phenotype analysis based on a large number of morphometric character indices and meristic counts can be used to discriminate fish populations up to the intraspecific level with the same results as the genotype analysis based on random amplified polymorphic DNA (RAPD) fingerprinting. Hence, the differences observed in phenotypic characters of the three O. niloticus populations in the present study based on the analysis made using 26 morphometric character indices, 8 meristic counts and four other morphometric characters indicates that the differences have also potential genetic bases.
Based on the parameters considered for characterization, Nile tilapia (O. niloticus) populations of lakes Chamo, Koka and Ziway differed in many phenotypic characters in their natural environments. As desired characters in aquaculture production, tilapia population of Lake Chamo had higher mean weight and length than population of Lake Koka followed by population of Lake Ziway.
The phenotypic characters are governed by genetic and/or environmental factors. Thus, it is important to confirm whether the populations could maintain these characters when grown under controlled environments.
Citation: Endebu M, Getahun A, Tessema M (2021) Differences in Phenotypic Characters of Nile Tilapia (Oreochromis niloticus L) in Three Ethiopian Rift Valley lakes; Screening Strains for Aquaculture. Fish Aquac J. S1:001
Received: 07-May-2021 Accepted: 21-May-2021 Published: 28-May-2021 , DOI: 10.35248/2150-3508.21.s1.001
Copyright: © 2021 Endebu M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.