Journal of Physical Chemistry & Biophysics
Open Access
ISSN: 2161-0398
ISSN: 2161-0398
Research Article - (2013) Volume 3, Issue 1
Specific hydration induced coil-globule transition in gelatin-B(GB) chain, a polypeptide, in ethanol, ethylene glycol and glycerol solutions (organo-solutions) was studied by Raman spectroscopy, viscosity and dynamic light scattering techniques to map its differential hydration behavior in mono, di and tri-ol solutions (alcohols are non-solvents for this polypeptide). The chain stiffness, determined from the hydrodynamic radius to radius of gyration ratio Rg/Rh, was estimated to be around 0.67 in water which was found to be dependent on the nature of alcohol and its concentration. This clearly attributed same random coil conformation to GB in all thesolutions at low alcohol concentration, but chain collapse to a near-globular shape (Rg/Rh=0.77) was observed when alcohol concentration was increased which was found to occur in glycerol at ≈ 60% (v/v), in ethylene glycol at ≈ 35% and in ethanol at ≈ 40% (v/v) concentrations consistence with the intrinsic viscosity data. The Huggins (KH) and Kraemer (KK) solute-solvent interaction parameter determined from the concentration dependence plot of relative viscosity revealed poorer interaction between GB and organo-solvents with increase in concentration of the organic phase. Differential chain hydration was evaluated from characteristic Raman active modes of water molecule. Three signatures Raman peaks were observed at 3200, 3310, and 3460 cm-1 specific to structured, partially structured and amorphous water respectively. The peak at 3310 cm-1 was observed to decrease with alcohol concentration while the peak at 3200 cm-1 was observed to increase indicating the increase in the water structure in all the solutions and depletion of water density near the first hydration sheath of the protein molecule which caused GB chain to collapse.
<Keywords: Protein hydration; Raman spectroscopy; Coil-globule transition; Protein conformation
Hydrogen bonding liquids and their mixtures occupy a special place among complex systems due to the existence of directed H-bonds. In contrast to covalent bonds, the H-bonds can be rearranged relatively easily. Although an enormous amount of literature exists which relates to the investigation of H-bonding systems, particularly on glycerol water mixture systems [1-7], there is still a lack of clear understanding even of the level of dynamics in “simple” water or alcohols. Among them, alcohols and their mixtures with water are widely used as excellent models to study cooperative dynamics. A variety of physical phenomena owe their origin to the specificity of the first hydration sheath found around a biopolymer which includes protein folding denaturation, DNA condensation and coil-globule transition etc. [8-11]. In all these processes, the hydrophobicity of the solvent environment and the hydrogen bonding capacity of the solvent with its co-solvent partner plays a decisive role. A systematic way to study the same can be attempted through appropriate tuning of solvent hydrophobicity and its hydrogen bonding capability by choosing alcohol solutions as solvent and a known biopolymer as probe molecule. Herein, we have chosen ethanol, ethylene glycol and glycerol solutions to observe how they affect the hydration of gelatin B (GB) biopolymer.
Gelatin is one of the most versatile and used gelling agents in food industry. It has found many applications in the pharmaceutical and photographic field [12,13] as it is an abundant raw material, produced all over the world at low cost and with excellent film forming properties [14]. Gelatin is obtained by thermal denaturation of collagen, which is the most common protein in the animal kingdom and consists of three polypeptide chains, each one twisted in a left-handed helix and supercoiled together to form a right-handed triple helix [15]. There are two main types of gelatin. Type A is extracted from collagen by acid treatment while Type B is extracted through the alkaline route. Type A gelatin is acid processed and has an isoelectric pH, pI≈9, whereas the alkali processed type B gelatin has pI≈5.2 we have used gelatin-B in this study. Gelatin is a polyampholyte molecule that makes the net charge on the molecule strongly dependent on pH. During the denaturation process the triple helix structure is broken to form a random coil gelatin chain. In aqueous solutions, the chains coil around each other to form a collagen-like triple helix when the solution is cooled below its helix-coil transition temperature [16]. In this way a three dimensional network is created. If the solution concentration exceeds 2% (w/v), the system transforms into a gel, which is a soft solid well known in food, cosmetic and pharmaceutical applications. If the protein concentration is much higher than 10% (w/v) the system is in general aimed to be used asa film for capsule formation.
In this paper we examine the hydration of Gelatin B in three different alcohol solutions i.e. ethanol, ethylene glycol and glycerol. The water content of these organo-solutions was varied in order to observe its effect on protein conformation which in turn is governed by the content of the first hydration sheath. The experimental work consisted of Raman Spectroscopy measurements which examined the role of binary solvent in gelatin dissolution and the concomitant change in the water characteristics. Viscosity measurements helped to find the intrinsic viscosity, interaction parameter and conformation of the biopolymer chain. Light scattering studies assigned explicit size to these molecules. We present a systematic and comprehensive hydration study of gelatin chain in three different and distinct alcoholic environments and show the resulting conformational transitions.
Gelatin-B (bovine skin extract, 225 bloom) having molecular weight 50 KDa bought from Sigma-Aldrich, USA was used as received to prepare the samples. The Bloom number indicates the amount of pressure, in grams, required to break the surface of a 6.67% (w/v) gel; higher Bloom number indicates higher gel strength. Triple deionized water from Organo Biotech Laboratories, India, was used to prepare the solutions. Seven solvents of water (100 - x) – solvent(x) binary mixture were prepared with concentration of solvent x (% v/v) varying from 0 to 60. Three different solvents were used, ethanol (EOH), ethylene glycol (EG) and glycerol (Gly). The solutions were prepared by dissolving gelatin powder in the required solvent with concentration set at 0.1% (w/v) and these were allowed to homogenize by resorting to continuous stirring for 1 h at 50°C. The stock solution had concentration well below gelation concentration of this biopolymer which is ≈ 2 % (w/v). A small quantity of sodium azide (NaN3) was added to the samples to prevent bacterial contamination. The samples appeared optically clear and transparent to the eye and did not contain air bubbles. These were stored in an airtight borosilicate bottles at room temperature (20°C). All the experiments were carried out within 48 hours of sample preparation.
Viscosity values were measured using a vibro viscometer (model SV10, A and D Co., Japan). This instrument uses a matched pair of gold-plated flat electrodes. The mechanical vibrations (frequency ≈30 Hz) set in one of these propagate through the sample and is picked up by the other electrode. The viscoelastic properties of the sample are deduced from the response function through the software provided by the manufacturer. Raman spectra of all the solutions were recorded on a FT-IR/Raman spectrometer attached with a microscope (Varian 7000 FT-Raman and Varian 600 UMA). We adopted Raman spectroscopy to investigate structure of water in alcohol solutions and gelatin because vibrational spectra are very sensitive to the local molecular environment.
Both static light scattering (SLS) and dynamic light scattering (DLS) measurements were carried out on gelatin in water-alcohol solutions to evaluate its chain dimensions. DLS experiments were performed at a scattering angle of θ = 90° and laser wavelength of λ = 632.8 nm on a 256-channel Photocor-FC (Photocor Inc., USA) that was operated in the multi-τ mode (logarithmically spaced channels). The goniometer was placed on a Newport (USA) vibration isolation table. The time scale spanned 8 decades, i.e., from 0.5 μs to 10s. Samples were housed inside a thermostated bath and the temperature was regulated by a PID temperature controller to an accuracy of ± 0.1°C. In all the experiments, the difference between the measured and calculated baseline was not allowed to go beyond ± 0.1°C. The data that showed excessive baseline difference were rejected. In this method, the system is physically probed over a length scale q-1 where q = (4πn/λ) sin θ/2. The laser wavelength in the scattering medium is λ/n, where n is index of refraction. The diffusion coefficient is related to corresponding apparent hydrodynamic radius Rh through Stoke-Einstein relation given as
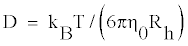 (1)
(1)
where solvent viscosity is η0, kB is Boltzmann constant, and T is absolute temperature. Details of DLS, and Guinier plot analysis used for determination of radius of gyration values, can be found elsewhere [17,18].
General formalism of structure of alcohol solutions
Recently, we proposed a hydration model and hypothesized that the specific hydration of oxygen atoms by available hydrogen atoms in the system dictates the area of various Raman bands [19]. Specifically, when the O-atom has all the 4 bonds (including the covalent and H-bonds) engaged with 4H-atoms, we describe it as fully structured, when 3 bonds are engaged with 3 different H-atoms, it is partially structured and when only 2 bonds are engaged with 2 H-atoms, we refer to it as free water molecule. The ratio of H-atoms available for hydrogen bonding to O-atoms in the system was calculated for various concentrations of glycerol and the same is shown in figure 1. For instance, it was shown that the hydration of Ow (O-atoms of water) increases while hydration of Og (O-atoms of glycerol) decreases with increase in concentration of glycerol in the solution. The hydration to the O-atoms is provided by the total number of H-atoms (HT) present. Thus, the ratios HT:Ow and HT:Og are relevant parameters that quantify hydration. It was clearly concluded that Ow contributes more to the ice-like structure and Og contributes predominantly to the partially structured water. Figure 1 data reveals HT:Ow ratio increases and HT:Og ratio decreases in the system with rise in glycerol concentration. Near 60% (v/v) glycerol concentration, one observes that the ratios HT:Ow and HT:Og are equal and their sum is minimum predicting that here the system has minimum hydration. At this point, one finds the freezing point minimum in the glycerol-water mixture. Such a simple concept sufficiently and universally described freezing point minima in ethylene glycol, methanol, ethanol, iso-propanol and glycerol solutions [19] (Figure 1).
Figure 1: Plot of Hydrogen atom to Oxygen atom (H:O) ratio shown as function of glycerol concentration. HT and OT represent total number of H-atoms and O-atoms available for H-bonding respectively, Ow and Og represent total number of O-atoms in water and glycerol respectively. Note the freezing point is minimum at 60% v/v glycerol concentration where HT:Ow + HT:Og is minimum [19].
Chain conformation
Gelatin is soluble in water, but not in any of the alcohols. Thus, the aqueous alcohol solution is a marginal solvent for this polypeptide. The measured normalized hydrodynamic radius (Rh) and radius of gyration (Rg) is plotted as a function of solvent concentration in figures 2-4 which indicate that the chain size is sensitive to propensity of hydrogen bonds that can be formed between the organic solvents and water. The number of hydroxyl groups is least in ethanol, it increases to two in ethylene glycol and it’s maximum in glycerol. Thus it was observed that as the number of hydroxyl groups increased in the solvent, the physical size of the polymer decreased considerably. This chain collapse is attributed to the changed hydration environment of the molecule (Figure 2).
The radii values were found to reduce by about 50% for glycerol, 35% for ethylene glycol and 30% for ethanol solutions. Interestingly, the chain collapse was distinctively seen where hydrogen bonding was maximum between the two liquids (freezing point depression point). More specifically, this occurred when glycerol, ethylene glycol and ethanol concentrations were close to 60, 35 and 40% (v/v) respectively. CT-DNA has been reported to exhibit condensation at ethanol concentration ≈ 42% (v/v) [20] (Figures 3 and 4).
The three dimensional conformation of the chain can be ascertained from the Rg/Rh ratio. For a random coil polymer this ratio is ≈ 0.67 whereas for a globular shape it is ≈0.77. This data is plotted in figures 2-4 which is very revealing. It is interesting to observe that this ratio remained invariant (0.65 ± 0.04) of alcohol type and their concentration in the concentration range 0-30% (v/v) and sudden chain collapse was seen for concentration > 30% (v/v). Thus, though the spatial stretch of the chain was reduced in water solutions of alcohols, their conformations did not alter for lower alcohol concentrations. It should be noted here that hydrophobicity of the nonsolvent though was found responsible for causing reduction in solubility of the biopolymer, did not affect chain conformation. This ruled out any possible gelatin alcohol hydrophobic interaction.
Chain hydration
The samples were subjected to Raman studies in order to resolve the issue of hydration of the protein chain in alcohol-water environment. The Raman peaks were seen to occur in two distinct frequency bands [21-23]: 600-2000 and 2800-3800 cm-1. The first frequency band exhibits identical peaks that could be superimposed exactly. These bands arise from various vibrational twists, and bending modes associated with C-OH and CH2 groups present in the organic molecules. The OH stretch modes are found in the frequency range 2800-3800 cm-1 which needs to be discussed in depth. Three signature peaks are located at 3200, 3310, and 3460 cm-1. The peak around 3200 cm-1 arises from in-phase vibration of OH stretching mode, structurally arranged water, which is sometimes referred to as ice-like structures. We observed a strong peak around 3310 cm-1. It can be attributed to the OH stretching of partially structured water molecules (liquid-like water). The peak around 3460 cm-1 arises from poorly H-bonded water molecules (amorphous water). The raw water spectra could be fitted to more than three Gaussian-Lorentzian functions.
The fractional area of the aforesaid Raman bands explicitly indicates the relative propensity of various structures under a given experimental condition. This data is depicted in figures 5-7. Note that abundance of amorphous water (3460 cm-1) is at best not more than 10% of the total. Thus, the structured and partially structured components governed the hydration of the GB chain. Figure 5 illustrates the systematic rise in structured water abundance with alcohol concentration with clearly distinguishable inflection observed at ≈ 40%, 35% and 60% (v/v) of ethanol, ethylene glycol and glycerol concentrations respectively. Correspondingly, a sharp dip was observed in the fractional area plot for partially structured water band (3310 cm-1) exactly at the above mentioned organic phase concentration. This clearly indicates the following: presence of alcohols helps in enhancing the water structure and at characteristic concentrations of the organic phase an abrupt change in the abundance of water structure was prevalent. Thus, the data shown in figures 5 and 6 are complementary. It needs to be emphasized that ethanol, ethylene glycol and glycerol do form hydrogen bonds selectively with water and the propensity of the same is maximum exactly at ≈ 40%, 35% and 60% (v/v) of ethanol, ethylene glycol and glycerol concentrations respectively (Figures 5-7).
Due to the preferential formation of hydrogen bonds between the binary liquids, the biopolymer chain finds itself in a marginal solventlike environment, the degree of which increases with rise in alcohol concentration. Due to this the first hydration sheath around the chain experiences gradual change. Partially structured water populates this hydration sheath primarily by forming hydrogen bonds with the acidic and basic residues of the protein chain. As more structured water is formed, the abundance of this water reduces leading to dehydration of the chain and the concomitant chain collapse. There is an organic phase depletion region close to the chain surface. This is illustrated schematically in figure 8.
Viscosity of solutions
Polymer solution viscosity is an important physical property in polymer research, development, and engineering. When high molecular weight nonionic polymer molecules dissolve in a fluid, they typically expand to form spherical coils. In dilute solutions, the volume associated with each polymer coil contains one polymer molecule surrounded by a much larger mass of solvent. A polymer coil’s hydrodynamic volume depends upon the polymer molecular weight and its thermodynamic interaction with the solvent. Polymer– solvent interactions depend upon the polymer molecular structure, chemical composition, solution concentration, solvent molecular structure, and the solution temperature.
Intrinsic viscosity [η] is the viscosity of an infinitely diluted polymer solution. Itis a measure of the hydrodynamic volume occupied by a macromolecule, which is closely related to the size and conformation of the chain, but is independent of concentration of macromolecule. In dilute solutions, by definition, the polymer chains are separated and there is negligible interaction between them. Therefore, the [η] of polymer in solution depends only on the dimension and the molecular weight of polymer chain. Experimentally determined values of the relative and specific polymer viscosities were used to calculate it, according to Huggins [24] and Kraemer [25] equations given by
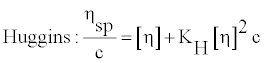 (2)
(2)
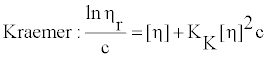 (3)
(3)
where c is the concentration of polymer solution, KH is the Huggins coefficientand KK is the Kraemer coefficient and theoretically it has been shown that(KH − KK)= 0.5.
The variation of intrinsic viscosity and the interaction parameters as function of organic phase concentration is shown in figures 9 and 10. The relative difference (KH − KK) is plotted in figure 11. These figures are quite revealing. Alike the radius data, the intrinsic viscosity value exhibited sharp decrease at well defined concentrations of alcohol (Figure 9).
The values of the KH and KK were observed to be decreasing with alcohol concentration and sharp change in their value was noticed at ≈ 60%, 35% and 40% (v/v) of glycerol, ethylene glycol and ethanol solutions (Figure 10). Interestingly, the size and hydration data of chains exhibited qualitatively similar behavior at identical organic phase concentrations. This is also in agreement with the Rg/Rh ratio data. The reducing values of the coefficients KH and KK indicated lesser repulsive force between chain segments that facilitates chain collapse at threshold alcohol concentrations (Figures 9-11).
The data in hand allows us to present the summary of the results through a schematic diagram illustrated in figure 8.
The three alcohols used in this work as solutions in water were observed to have remarkable effect on gelatin chain conformation. A clear identifiable coil-sphere conformational phase transition was detected at the characteristic alcohol concentration. And exactly at this concentration the binary solvent exhibited freezing point depression and maximum inter solvent hydrogen bonding. The contraction in chain volume was maximum for glycerol, intermediate for ethyl glycol and minimum for ethanol. The propensity of hydrogen bonds is maximum in glycerol solution followed by ethylene glycol and ethanol. Raman data helps to explore the water structure involved in solution phase. In our proposed hydration model, the ice-like water structure is contributed by the oxygen present in water molecule while the liquid like structures are contributed by oxygen atoms of both alcohol and water molecules. In the binary solvent system the probability of formation of ice-like structure by O-atom in water molecule increases due to availability of more hydration to it, but such O-atoms decrease in number with increase in alcohol concentration. In solution phase, since the number of gelatin molecules is very less, the contribution of O-atoms present in gelatin to alter the structure of the system is very less if the H:O ratio is considered. The polyampholyte nature of gelatin plays a major role by formation of hydration layer around it which contributes mainly to the ice-like structure. On increase of alcohol concentration, this ice-like structure in the hydration sheath is disturbed by alcohol molecules; hence an increase in propensity of the same is seen. This forces the chain to collapse. Timasheff [26] has extensively reviewed the effect of glycerol solution on protein hydration. More experiments need to be done with other biopolymeric sols and gels in organic solutions to generate a universal understanding of hydration of organogels vis a vis water structure.
We are thankful to Advanced Research Instrumentation Facility of the University for allowing us access to Raman facility. This work was supported by a grant from Departmentof Science and Technology, Government of India.