
Biochemistry & Pharmacology: Open Access
Open Access
ISSN: 2167-0501

ISSN: 2167-0501
Case Report - (2024)Volume 13, Issue 3
The Syndrome of Inappropriate Anti Diuresis (SIAD) is a rare and significant side effect of a variety of medications such as antidepressants, chemotherapeutic medications, and non-steroidal anti-inflammatory drugs. Regarding Dipyrone, only a few cases have been mentioned in the world. The non-opioid analgesic metamizole (Dipyrone) is used for the treatment of acute or chronic pain and fever and is one of the most frequently prescribed over-the-counter medications in Israel, Brazil, and Mexico. Dipyrone has low anti-inflammatory properties yet inhibits prostaglandins to some degree. In this case report, we present a case of a post-operative patient, who was on an analgesic cocktail containing Dipyrone. The patient developed a new and sudden hyponatremia, namely, a drop of 12 meq/l of sodium in less than 24 hours. Dipyrone was found to be the culprit drug. Our case report demonstrates the importance of considering Dipyrone in the diagnosis of medication-induced SIAD in patients taking analgesics.
Dipyrone; Metamizole; Hyponatremia; Hip fracture
The Syndrome of Inappropriate Anti Diuresis (SIAD) is a possible cause of hyponatremia characterized by excessive free water retention and impaired water excretion. SIAD can be caused by neurological diseases, pulmonary diseases, neoplasms, mostly by Small Cell Lung Cancer (SCLC), and a variety of drugs, such as anticonvulsants, antidepressants, chemotherapy, Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), and opiates [1]. The incidence of SIAD caused by NSAIDs has rarely been reported in the past century [2-5], yet the mechanism of the NSAID drugs’ action is very clear. NSAIDs enhance the action of Anti-Diuretic Hormone (ADH) at the renal tubule level by prostaglandin inhibition [6]. In this case, we witnessed an event in a patient who was admitted to the hospital for a hip fracture. The patient was treated with a combination of drugs, which included tramadol, oxycodone and Dipyrone as analgesics. Within 24 hours, he developed a new onset hyponatremia secondary to Dipyrone.
A 78-year-old male was admitted to the Emergency Department (ED) due to a hip fracture secondary to a sudden fall in his home several hours before his admission. The medical history was as follows: Diabetes mellitus, hypertension and glaucoma, all of which started several years prior to the current admission. His drug list consisted of the following: Metformin 850 mg once per day, Glibenclamide 5 mg once per day, and aspirin 100 mg once per day. The patient had been taking all his medicine appropriately and all diseases were well controlled.
Upon arrival to the ED, his vital signs were as follows: Temperature 36.4°C, blood pressure 167/86 mmHg, pulse 79 bpm, 14 breaths per minute without hypoxemia. A pelvis X-ray was performed and demonstrated an intertrochanteric fracture of the left femur. The patient was then transferred to the operating room where he underwent closed reduction with internal fixation of the femur via insertion of percutaneous compression plating. The surgery went well without any immediate post-operative complications except for a 2 gm reduction of hemoglobin levels (from 9 to 7 g/dl), which was treated with one intravenous red-packed cell infusion.
Once patient was hemodynamically stable, patient was transferred to the orthopedic surgery department, where patient was treated with analgesics, including Tramadol 100 mg once per day, Oxycodone 9 mg once per day and Dipyrone 500 mg four times per day, in addition to his usual medications as listed above.
On his first day of admission to the Department of Orthopedic Surgery, the patient started to complain of dizziness and vomiting. Vital signs were within normal range, and a physical examination did not reveal any exceptional findings. Blood analysis demonstrated an acute drop in sodium levels from 132 meq/l to 120 meq/l in less than 24 hours as shown in Figure 1. Moreover, the blood-osmolality level was 264 mosm/kg, urine-osmolality and urine-sodium levels were 827 mosm/kg and 71 meq/l respectively, consistent with the diagnosis of true hyponatremia secondary to SIAD.
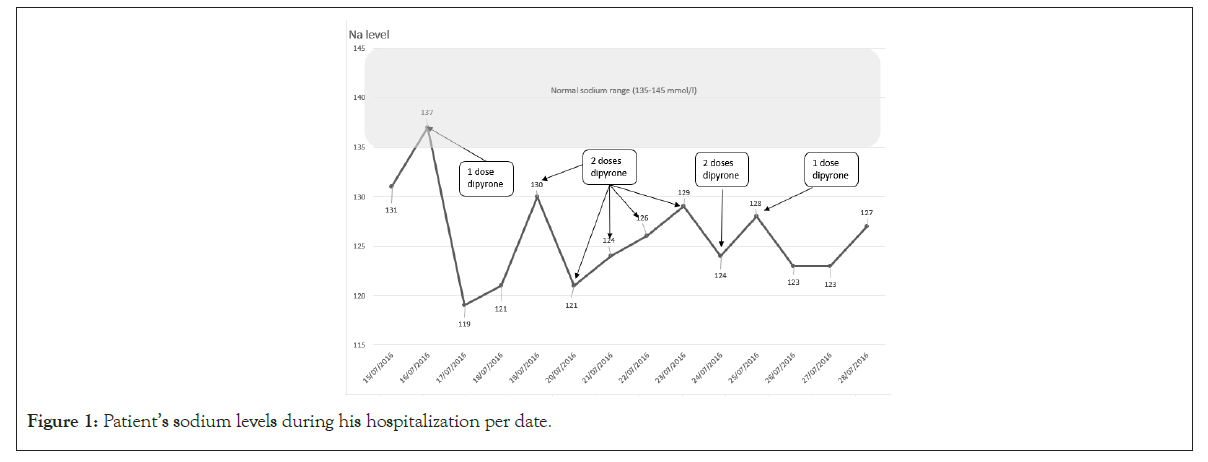
Figure 1: Patient’s sodium levels during his hospitalization per date.
Given the acute onset of SIAD, the patient was transferred to the Department of Internal Medicine, where he was treated with fluid intake restriction and elimination of all of his analgesics. The sodium level rose to 130 meq/l within the next 24 hours. Patient was then transferred back to the Department of Orthopedic Surgery, where the treatment for pain management was continued with Targin (Oxycodone/Naloxone) and Dipyrone, in the same regimen mentioned above. Two days later, the blood analysis once again demonstrated an acute hyponatremia of 121 meq/l with laboratory findings consistent with the previous SIAD. As a result, malignancy was suspected as a cause of the SIAD, and a total body Computed Tomography scan was performed, which revealed no evidence of any space-occupying lesion. Since Oxycodone was the main suspect for the drug-induced SIAD, it was eliminated from the pain management regimen and the patient only continued taking Dipyrone 500 mg four times per day. However, hyponatremia persisted with fluctuating values. Finally, Dipyrone was eliminated, and sodium levels rose to 128 meq/l.
Before his discharge, a drug challenge of a single dose of 1 gm of Dipyrone was given. The sodium level dropped once again to 123 meq/L, rising back to 127 meq/L the following day, consistent with the suspected diagnosis of Dipyrone-induced SIAD. Since it was clear that eliminating the culprit drug was an efficacious treatment option for his hyponatremia, no additional treatment was given.
The patient was discharged with a recommendation not to take any kind of analgesic, including Dipyrone and for a routine checkup at our clinic to repeat the Dipyrone challenge test as shown in Figure 2. Eight days after his discharge, blood levels of sodium were normal at 137 meq/L. A week later, patient was asked to take a single dose of 1 gr Dipyrone. Sodium levels were measured at our clinic one and three days subsequently; sodium levels dropped acutely towards 131 meq/l and 128 meq/l, respectively. To ensure the normalization of sodium levels in the absence of the Dipyrone effect, blood tests were drawn once again six days later and demonstrated a value of sodium 134 meq/l, concluding a definitive diagnosis of Dipyrone-induced SIAD.
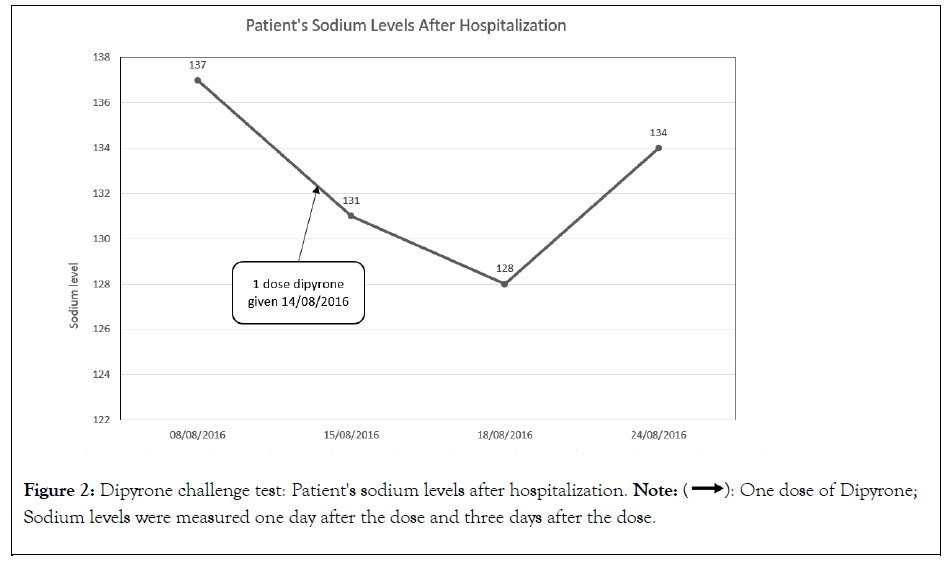
Figure 2: Dipyrone challenge test: Patient's sodium levels after hospitalization. Note:  One dose of Dipyrone; Sodium levels were measured one day
after the dose and three days after the dose.
One dose of Dipyrone; Sodium levels were measured one day
after the dose and three days after the dose.
SIAD is characterized by excessive free water retention and impaired water excretion, causing euvolemic hyponatremia. SIAD can clinically manifest with abnormally high thirst, dizziness, and confusion. SIAD can mostly be caused by SCLC and drug therapies. The main medications that have shown implications in this syndrome are the antidepressants [1]. Other drugs worth mentioning are Cyclophosphamide, Vincristine, Vinblastine, and opiates [6]. Opiates are known to have a twofold increased risk of hospitalization for hyponatremia [7]. All the aforementioned medications can cause an inappropriate increase in ADH [1], even though some may potentiate the effect of this hormone at the level of the kidneys [8]. Another noteworthy reason that might have caused SIAD is post-operative stress, which is rare but has been reported previously [9]. The post-operative state has long been known to be characterized by avid renal sodium and water retention. Non-osmotic release of the posterior pituitary ADH, which acts on the collecting duct and results in renal water retention, appears to be at least in part responsible for renal water retention [10].
The association between SIAD and NSAIDs is rare and has been reported by several studies over the past seven decades [2-7]. NSAIDs enhance the action of ADH at the renal tubule level by prostaglandin inhibition [6]. Renal water reabsorption depends on the action of ADH, which is mediated by cyclic Adenosine Mono Phosphate (cAMP). Medullary prostaglandins have an inhibitory action by decreasing cAMP production. Dipyrone, like Indomethacin, Aspirin, and Ibuprofen, is an inhibitor of prostaglandin synthesis. Therefore, NSAIDs may trigger an increase in cAMP, which in turn will cause an increase in the action of ADH and water retention. It is important to note that over the years, some cases have suggested that this is not the case with every NSAID [3-4].
Similar to Brazil, Mexico, Russia, and Spain, Dipyrone is one of the most commonly prescribed over-the-counter analgesic medications in Israel [11]. In the USA, France, and Australia, Dipyrone has either not been approved or has been withdrawn from the market due to safety concerns, particularly regarding the risk of agranulocytosis [12].
The majority of cases involving NSAID-induced SIADH were attributed to ibuprofen, typically managed through fluid restriction and discontinuation of the drug. One case report demonstrated that the use of tolvaptan was an efficacious strategy in NSAID-induced SIAD [2].
It has been thought that SIAD is caused by the use of NSAIDs and mainly appears in elderly patients [4], but as seen in the case of Roche, et al. SIAD was presented in a young and healthy adult [6]. Even though such cases are rare, a thorough diagnostic process is essential to determine the cause of hyponatremia, which should also be considered in compiling the patient’s medication list.
In this case, we found Dipyrone to have caused the SIAD. This case emphasizes the importance of considering the uncommon yet severe side effect of Dipyrone when managing patients with acute or chronic pain, particularly in outpatient settings. Patients experiencing mild symptoms may not disclose their use of Dipyrone, which is easily obtainable as an over-the-counter medication. Therefore, it becomes obligative for healthcare providers to actively inquire about all medications, including over-the-counter drugs, to ensure comprehensive assessment and management of potential adverse effects.
It's imperative for clinicians to recognize the potential rare adverse effect of SIADH associated with Dipyrone usage, especially considering its widespread prescription for pain management. This highlights the importance of heightened vigilance and thorough patient education regarding the potential symptoms of SIADH. By increasing awareness among both healthcare providers and patients, we can facilitate early recognition and management of this serious complication, ultimately optimizing patient safety and outcomes.
Citation: Hindy J, Kravitz D, Rainis T, Greenberg D (2024) Dipyrone-Induced Inappropriate Antidiuretic Hormone Secretion: A Case Report. Biochem Pharmacol. 13:366.
Received: 04-Jul-2024, Manuscript No. BCPC-24-32647; Editor assigned: 08-Jul-2024, Pre QC No. BCPC-24-32647 (PQ); Reviewed: 23-Jul-2024, QC No. BCPC-24-32647; Revised: 30-Jul-2024, Manuscript No. BCPC-24-32647 (R); Published: 06-Aug-2024 , DOI: 10.35248/2167-0501.24.13.366
Copyright: © 2024 Hindy J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.